Although economically beneficial, traditional herd reproductive health programs may not
be structured to optimize income over reproductive costs in dairy herds. In addition,
subscription to reproductive herd programs has not prevented reduction in reproductive
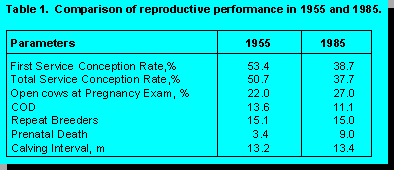
efficiency over the last 30 to 40 years. Dr. Zemjanis (39) compared performance in the
same herds in 1955 with that in 1985. All herds subscribed to a reproductive health
program. The results are in the following table:
Reproductive performance is worse in 1985 than 1955. The reproductive program had not prevented decline in conception rate (CR), increased calving interval (CI), or increased perinatal losses. Certainly it could be argued that increased production, herd size, and responsibilities for herd personnel could have resulted in even poorer performance had the herds not been on a reproductive program. However, the decline in reproductive performance in these herds is similar to that in herds on DHIA test service over the same period of time. A recent survey from the University of Illinois found that only 50% of DHIA herds subscribe to a reproductive herd health program. Thus, it might be expected that the decline in performance in DHIA herds would be worse than in the above subscription herds if declines in performance were mitigated by reproductive programs. This is not the case. Possibly technology developed and employed in 1955 is not optimal to deliver reproductive services in 1985 or 1994. After all, many methods employed to manage dairy herds have changed over the last 30 years. Maybe reproductive herd health programs need to progress beyond rectal sleeves and scheduled visits.
Reproductive programs can be beneficial to dairy managers because they can assure a majority of cows in the herd realize an optimal CI, cows are culled for desired reasons, labour is used efficiently for heat detection, cows with reproductive disease receive medical attention within regulatory guidelines, and semen is allocated to populations of cows economically. The veterinarian is an ideal person to coordinate, program, evaluate, and monitor herd reproductive performance. Too often, dairy producers have reduced their expectations of their reproductive program because they have not been able to meet optimal goals. Effective herd reproductive programs need to improve herd reproductive performance. Why have current reproductive programs been less than ideal? What constitutes an effective herd reproductive program? What is needed to maximize reproductive performance within dairy herds?
Reproductive Performance
Indices of HDR and CR are influenced by biology (cow factors such as disease and nutrition), environment (heat stress, flooring), and management (accuracy and efficiency of heat detection, semen placement). Voluntary waiting period and breeding period are controlled by management and are adjusted to achieve acceptable proportions of pregnant cows each lactation cycle. Reproductive programs balance the influence of these factors on reproductive performance. Ideally, due to the shape of the lactation curve, reproductive programs should strive for high HDR and CR and short breeding periods with pregnancy proportions of 85% or higher by 120 to 140 days in milk.
A farm has limited resources to allocate towards controlling reproduction. Resources
should be allocated to have the largest possible effect on herd performance with the
minimum input. Reproductive indices do not have equal weights effecting reproductive
performance. Heat detection may be divided into that for first insemination (PREHDR)
and for repeat insemination (POSTHDR). Effects of VWP, PREHDR, POSTHDR and CR
on calving interval, culls for failure to become pregnant over a 210 day breeding period,
and percent of cows calving pregnant prior to 115 days is presented in the following table.
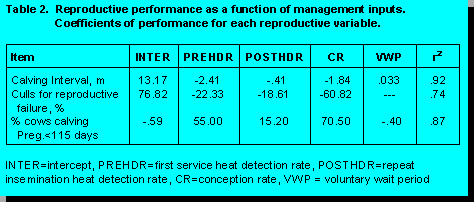
Each indices influences reproductive performance with unequal weight. For example, CI is reduced .33 months for every 10 days earlier VWP; CI is reduced .241 months if PREHDR is increased from .50 to .60; CI is decreased .041 months if POSTHDR is increased from .50 to .60; and CI is decreased .184 months if CR is increased from .50 to .60. Improvement in PREHDR influences CI more than improving POSTHDR, thus resources should be allocated to these differently. First service heat detection rate and CR are the primary variables influencing CI, calves born per year and culls for reproductive failure. Repeat insemination heat detection (POSTHDR), conception to second and later services, and breeding period have small influences on calves born per year and culls for reproductive failure. Farm resources should be concentrated on PREHDR and CR to first service (FSTCR) to exert the greatest control on reproductive performance.
Pregnancy rate (PR) represents the proportion of open cows which become pregnant over a 21 day period. It is equal to HDR times the CR and describes open cows seen in heat and successfully inseminated. Mean CI for the herd, culls for reproductive failure, and calves born per year are highly correlated with PR - .89 to 80. PR captures both heat detection and conception in one variable and determines reproductive performance in the herd. The lower the PR, the longer the CI, the more cows culled for reproductive failure (2), and the fewer calves born per year. Pregnancy rate should be at a level to maximize returns from reproduction. This is the variable we monitor most closely in our reproductive programs as it determines all our reproductive outcomes.
Identification of problem versus normal cows has been the backbone of veterinary reproductive health programs. Identification of problem and normal cows only will be beneficial if it improves PR within these groups of cows, either by increasing HDR or CR.
Traditional Herd Reproductive Programs
Uterine Condition
Typically, rectal examination is performed 15 to 40 days postcalving. Uterine involution is a dynamic process, therefore criteria for assessing normality should change with days postcalving at examination. Involution is a process of reduction in size and endometrial repair, which occur through muscular contraction, vasoconstriction, loss of tissue (necrosis and sloughing), and endometrial regeneration. The process is usually complete by 30 to 50 days postcalving. Involution is most rapid from 10 to 20 days postcalving, which is evident from the copious amount of fluid (lochia) discharged during this period, particularly from 14 to 18 days postcalving. Involution is delayed in older cows, cows with parturient disorders (dystocia, milk fever, retained placenta, and metritis), cows with twins, and cows calving in the winter (1, 15, 17, 18, 25, 31). Histologic repair usually lags physical involution by 10 to 20 days or more (32).
Abnormal postpartum cows are identified based on uterine horn size and texture in addition to vulvar discharge. Cows identified as abnormal are treated, which usually has been an intrauterine infusion of antibiotics, antiseptics, chemical irritants, prostaglandin injection, or a combination of agents (3, 23, 27, 29, 31, 36, 37). Abnormal ovarian structures are classified and treated if cystic ovarian disease (COD) is diagnosed (7, 19, 21). Cows are then reassigned for re-examination at a future herd visit.
Uterine infection is common postcalving, because uterine involution is a septic process, however, illness is not common. Griffen et al. (16) found that uterine infection was a function of week postcalving. In cows with normal parturition and no postpartum complications, 92% of cows cultured positive in the first week postcalving, 96% in the second week postcalving, 77% in the third week postcalving, 64% in the fourth week postcalving, 30% in the fifth and sixth weeks postcalving, and 25% in seventh week postcalving (16, 17). Most infections were a mixed bag of coliform, streptococcus, and staphylococcus species.
Cows become sick in the first two weeks postcalving when Actinomyces pyogenes and anaerobic organisms (Fusobacterium species and Bacteroides species) and Clostridium species overwhelm uterine defenses; acute metritis ensues (16, 17). Predisposing conditions include extremely virulent organisms, overwhelming challenge, and poor immune function. Acute metritis is fairly easy to recognize: cows are febrile, off feed, and have a fetid watery discharge. These cows need to be treated with systemic antibiotics, preferentially penicillin injection in the muscle at 10,000 to 20,000 IU/lb twice a day for at least three days. In addition these cows may need supportive care, such as fluids and antiphlogistics.
Failure to clear Actinomyces pyogenes from the uterus beyond three weeks postcalving results in chronic endometritis and reduced conception rate if the infection is severe enough (22, 29, 38). From 30 to 100% of cows with acute metritis will develop chronic endometritis. Very severe infection may develop into a pyometra, preventing estrous cycles and causing severe endometrial damage. From 5 to 30% of cows with no abnormal history postcalving may develop chronic endometritis. Identification of these cows is difficult as they may have no overt signs of problems and have no history of problems around calving (25). Cows with acute metritis and abnormal calving events are prime candidates for developing chronic endometritis and can be identified from historical records and treated accordingly. However, it is difficult to identify chronic endometritis cows from the population with normal parturitions. Postpartum palpation has been advocated as a screening test to identify cows with endometritis from normal cows and to prescribe corrective therapy and return these cows to normal fertility (25).
Although weakly correlated with uterine culture and biopsy (32), rectal palpation is a poor diagnostic test to indicate chronic metritis (25). Tennant et al. (33, 34) and others have found that size alone is a poor predictor of infection and fertility. Size of the uterine horns between 30 to 50 days postcalving had no association with fertility (34). In our studies, size and size differential of uterine horns and/or cervix was not a useful predictor of first service CR. Thus, palpation alone was not apparently a strong predictor of problem cows (32, 34).
To improve diagnosis of problem cows, it has been recommended to use rectal palpation in combination with vaginal speculum exam of cervical discharge or speculum exam alone (22). Miller et al. (22) found that twice as many cows classed as infected on speculum exam had positive cultures compared to cows classified as infected using rectal palpation alone (59% versus 22%, respectively). Studer (32) found gross palpation of size weakly associated with bacterial culture and histology, whereas discharge had a higher correlation with culture and histology. Selection for treatment Studer felt required multiple criteria based on palpation and examination of vaginal tissues and cervical discharge on speculum exam. However, even using this criteria results in too many false positives and false negatives.
Most data suggest that other than for identifying pyometra, postpartum palpation is a poor screening test for identifying abnormal cows and a poor predictor of future fertility. Several reasons exist for this. For example, time of exam postcalving will influence the predictive value of palpation for identifying infection. If palpation is done at 14 to 28 days postcalving many cows will be identified as infected (false positives) due to the high incidence of infection and pus at this time postcalving. Callahan (3) estimated that metritis classification based on palpation and discharge would change from 30% to 8% just by changing time of exam from 14 days to 28 days (3). Many cows will normally clear any infection by 30 to 50 days, thus palpating earlier identifies many cows who would need no therapy. Furthermore, mild to moderate endometritis has little effect on fertility (17, 29). Cows with a mild to moderate endometritis may have reduced conception rates at first service, but normal rates at second and third service. For these reasons, identification of problem cows on rectal exam has little correlation with future fertility and thus has little diagnostic or prognostic value.
In addition, treatments used to correct endometritis have often been at best ineffective and at worst harmful to the uterus (29, 36, 38). Irritating solutions and some antibiotics may damage the endometrium, reduce natural uterine defense mechanisms, or be inactivated in the uterine environment, particularly the early postpartum cow (29). In two controlled trials, Thurmond et al. (36) found penicillin or oxytetracycline infusions had no effect on PR compared with control nontreated cows and normal cows. In fact, increasing PR was most beneficial for improving reproductive performance in these cows, not therapy. For these many reasons, postpartum palpation programs have been weak, ineffectual tools to improve herd fertility.
Chronic metritis can impair fertility (11, 12), as can other postpartum disorders (4, 5, 8, 9, 10). Intrauterine therapy for endometritis has been ineffective (3, 27, 36). Although odour may be diminished by intrauterine antibiotics, fertility was not returned to normal. Only prostaglandin (PGF) injection at 14 to 40 days postcalving has shown a benefit on fertility (23). Cows treated with PGF have had no difference in FSTCR compared with normal cows. Strongly irritating solutions, such as strong iodine solutions, nolvasan, furacin, may cause damage to the endometrium and reduce CR. These solutions should be avoided. We have not used uterine infusion as a therapy since 1983.
Ovarian Structures
Anestrous cows will have small inactive ovaries with little change over sequential examinations. Follicles <15 mm in diameter may be found on examination, but fail to ovulate. There is no effective treatment for these animals except time. If more than 10% of cows are anestrous beyond 40 days postcalving then dry cow, springing heifer, and lactating diets need to be closely examined for adequate nutrition.
Pregnancy Exam
Reproductive Economics
Most economic models suggest cows are more profitable when days open are under 120 days. Milk produced per day of herd lifetime is increased when cows have fewer nonproductive days in their total lifetime. Nonproductive days are minimized by reducing days open and age at first calving. Milk produced per day decreases about .35 kg/day for every missed heat and pregnancy opportunity beyond 50 days postcalving. Cumulative costs of heifer rearing increase about $40 (US) for every month beyond 20 months of age at first calving. Calves born per year decrease by 14% for every increase in 30 days open in the herd. Thus, for all reproductive efficiency measures, delayed pregnancy reduces income.
It is more effective to look at profit from reproduction as a function of reproductive
variables HDR and CR. Profit from reproduction is a function of pregnancy rate (PR), a
variable which combines HDR and CR (PR=HDR*CR). DAIRY ORACLER, is a program
developed by Dr. Will Marsh at the University of Minnesota, for calculating the effects of
reproduction on herd profit. Using inputs of milk January 3, 1995 price at $10.50, calf
value at $75, cull cows at $500, semen cost at 10.00/straw, and replacement heifer costs
at $1,000, gross margin per cow per year as a function of PR appears as follows:
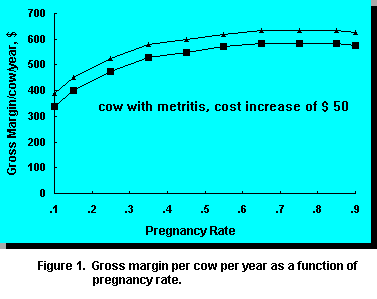
Profit increases with increasing PR from .1 to .45 and then begins to flatten. It is apparent that gross margin/cow is optimal when PR is .35 or higher. Higher PR only slightly increases gross margin/cow and thus may not be profitable if costs to increase PR from .35 to .45 are high. Improvement of PR above .35 should occur with less marginal investments/cow. Most dairy farms operate with a PR of .15 to .25, thus gross margin can be improved by $50 to $100/cow/year with improvement in PR to .35. The goal of the reproductive program should be to achieve a PR of .35.
Secondly, cows in the herd with increased health costs have lower gross margins due to increased veterinary, labor, and drug fees. Often these cows have low CR, which will lower PR, unless HDR is increased. Often these cows have lower returns (1, 4, 5, 8, 9, 10, 11, 12, 25). Such cows have lower marginal returns because of reduced reproductive efficiency and higher costs associated with treating the disorders. Herd programs should identify these groups of cows with reduced fertility so reproductive programs can be structured to realize optimal returns in these subfertile groups via differing strategies. Cows with poorer reproductive potential need to be identified and managed so costs to achieve pregnancy are reduced, because costs in this population are already increased. This means controlling semen costs in populations with potential for lower CR and managing HDR to off set lower CR.
Example of a Herd Program
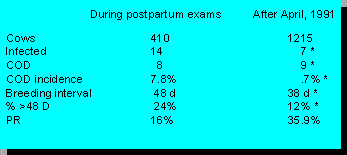
Cows infected at pregnancy exam, or after 40 days postcalving are lower since we began routine prostaglandin injections at 28 days postcalving. Cows with COD are reduced and PR is at our goal of 35% or higher. This herd has been able to sell replacements for dairy purposes and maintain a higher milking herd since we began this program than when they did weekly postpartum exams.
Conclusion
References