
Some other stresses slow plant maturation, which results in herbage quality being maintained at high levels for a longer time. This is illustrated by the effects of drought and insect damage on forages. Both cause a reduction in leaf mass. If the loss of leaves is not severe, these stresses may have little effect on forage quality or may actually improve forage quality. Forage grown under shaded conditions usually has higher crude protein concentrations than unshaded forage. Diurnal variation also exists in forage quality with highest quality in the late afternoon as a result of photosynthate accumulation, which dilutes cell-wall concentration and lowers NDF. Soil nutrients only have small effects on forage quality. Nitrogen fertilization usually raises the crude protein concentration of nonlegume forages. Foliar diseases and plant pests probably have the greatest adverse effect on forage quality by reducing digestibility. Some weeds reduce forage quality but many have nutritive values similar to forages and have little impact on forage quality.
Introduction
Forage environment includes numerous factors. Those that I will consider are temperature, drought, sunshine intensity and duration, soil nutrient deficiency, and pests. Environment often exerts its greatest influence over forage quality by altering leaf/stem ratios, but it also causes other morphological modifications and changes in chemical composition of plant parts including that of cell walls. Forage cell walls, composed mostly of polysaccharides (carbohydrates) and lignin, limit forage consumption (intake) and digestibility. Plant environment also influences senescence rates and amount of dead plant material, which affects forage quality. Senescing plant material is of lower quality than green tissue and grazing animals select for young, green leaf tissues rather than stem and dead plant tissues.
Temperature
The primary effects of temperature on forage quality are to determine the rate of plant maturation and to influence the relative proportions of leaves and stems. When harvested at a particular growth stage, highest yields are usually obtained when forages are grown under temperatures somewhat lower than those for optimal growth (8). When grown at temperatures above those optimal for growth, forages tend to be shorter at flowering and to bloom earlier than when grown under cooler temperatures.
The depressed digestibility associated with elevated temperatures is usually attributed to higher forage cell-wall concentrations, which is estimated by neutral detergent fiber (NDF). Additionally, the NDF of forages grown under warm temperatures is usually less digestible than that of forages grown under cool temperatures (3). During spring, the effect of warming temperatures interact with advancing plant maturity to cause a more- rapid decline in forage quality with time than occurs during the summer. Spring-grown forage can be of very high quality if harvested early, but because of the rapid rate of decrease in quality with time, delayed harvest or grazing will have a large negative impact on quality. Because the majority of forage from cool-season, perennial species is produced during the spring, harvesting and grazing per unit of land proceed at a slower rate than during the summer where yields are lower. As a result of the high spring yields and rapid decline in forage quality with time, mismanagement during the spring has a greater negative effect on total forage quality than occurs during the summer.
Drought
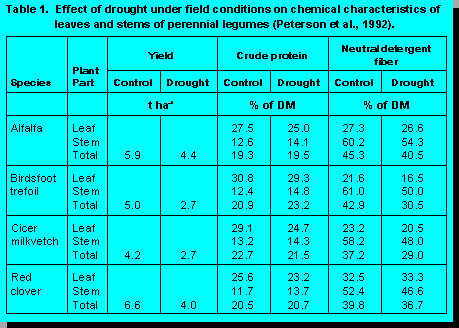 Photosynthetic rates are usually
affected less by drought than are respiration rates and growth, causing a general increase
in concentrations of highly digestible soluble sugars in forages (3). On the negative side,
however, the leaf/stem ratio can be reduced by acceleration of the rate of death of older
leaves. Leaves comprise a large portion of forage yield and they represent an even
higher proportion of total nutrients. Hence, their loss from plants can have an especially
adverse effect on forage quality.
Photosynthetic rates are usually
affected less by drought than are respiration rates and growth, causing a general increase
in concentrations of highly digestible soluble sugars in forages (3). On the negative side,
however, the leaf/stem ratio can be reduced by acceleration of the rate of death of older
leaves. Leaves comprise a large portion of forage yield and they represent an even
higher proportion of total nutrients. Hence, their loss from plants can have an especially
adverse effect on forage quality.
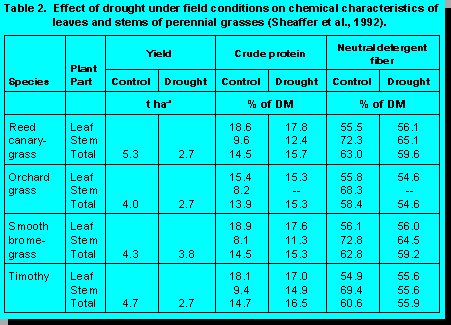
Reports regarding the effect of drought on CP concentration sometimes have been contradictory (3). Inconsistent results may be a function of the degree to which water stress causes leaf senescence and changes in the leaf/stem ratio. Additionally, drought effects on CP concentration may be determined by distribution of nitrogen in the soil profile in relation to location of the limited available soil water (3). If both nitrogen and water are present in the same soil horizon, they may be taken up together and forage CP concentration may be unaffected or may be increased if soil nitrogen is more available than water. If sub-soil water is ample and most of the soil nitrogen is near the surface, however, growth may continue with reduced nitrogen uptake so that forage CP concentration declines.
Sunshine Intensity and Duration
Variation in photoperiod can play an important role in induction of reproductive development of many forage species, and affects forage quality indirectly by causing plants to shift from vegetative growth to reproductive development. Reproductive development causes production of more stem material and leaf production slows in grasses, resulting in higher NDF concentrations.
Effects of Shading
Forages frequently are shaded. This occurs during periods of cloudiness, when forages are shaded by tall plants such as trees in pastures, or when top leaves and stems shade bottom leaves and stems. Shading has both direct and indirect effects on forage quality in that it alters the chemical composition of forages as well as morphological development and yield. Crude protein concentration is usually greater in leaves and stem segments from the top of plant canopies than from the bottom. This has been attributed to shading within the plant canopy, which enhances senescence rates of bottom plant parts (3).
Shading also reduces tillering of grasses and slows the growth rate of forages. It typically has a greater effect on forage yield than on quality. For example, Kephart et al. (17) and Kephart and Buxton (16) found that imposing 63% shade on five perennial forage grasses reduced yield by 43%, but only reduced NDF concentration by 3% and increased digestibility of the forage by 5%. Samarakoon et al. (24, 25) also found that digestible DM of grasses grown under shade was somewhat higher than that of herbage grown in full sunlight when fed to animals.
Crude protein concentration is much more responsive to shading than other quality characteristics. Kephart and Buxton (16) found that 63% shade increased CP concentration by 26% in forage grasses. The responses were generally greater for leaf blades than for stems. Increased concentration of nitrogenous compounds from shading is usually at the expense of soluble carbohydrates. The CP response for legumes from shading is generally smaller than for grasses. This may occur because nodulation and nitrogen fixation of legumes are restricted by shade.
Reduced NDF concentration from shading has not been reflected in increased DM digestibility in all studies. While there is some conflict as to whether shading has a positive or negative effect on digestibility, the effects reported have generally been relatively small (3). Some of the differential response could result from variation in plant species investigated, influence of other environmental conditions, differing effects on leaf/stem ratio, and the length of time forages were shaded.
Forage quality undergoes diurnal variation with highest quality in mid afternoon. Water- soluble carbohydrate concentration in forages was shown to follow a pattern of lowest values before sunrise and highest values in the afternoon (12). Lechtenberg et al. (18) likewise found digestibility of alfalfa was 1.6 percentage units greater in the late afternoon than in the early morning before sunrise. Starch concentration in leaves increased by 10 percentage units during the daylight, whereas that in stems did not change. These changes caused simultaneous shifts in the leaf/stem ratio from 1.1 to 1.5. Additionally, protein tends to be degraded at night through proteolysis followed by remobilization of nitrogen and protein synthesis during daylight. The result is fluctuation in protein concentration of 5 to 15% over a diurnal period (3).
Effects of Photoperiod
In Canada, compositional changes of spring-grown forages during maturation reflect the influence of both warming temperature and lengthening photoperiod. During summer regrowth, however, both temperature and photoperiod show little seasonal change as forages grow and mature.
Both daylength and sunshine intensity influence morphology, growth, flowering, and maturity of forages. When daylength satisfies the photoperiod requirement of photoperiod-sensitive species, plant development changes from vegetative growth to reproductive development. The more the photoperiod requirement is satisfied, the greater the reproductive expression. Heide (11), for example, noted a three-fold increase in average timothy stem height as photoperiod increased from 8 to 24 hours.
Aside from the effects on flowering, long photoperiods tend to cause high forage quality because of greater photosynthetic activity, which in turn increases soluble sugars that dilute the NDF. Work summarized by Deinum et al. (5) indicates that each one-hour increase in daylength increases digestibility by about .2 percentage units. Yield also increases and plant morphology is usually altered under long photoperiods. Shoot/root ratios generally increase and leaf/stem ratios may decrease as daylengths are extended. Juan et al. (15) reported that alfalfa grown under 13-hour photoperiods had a higher leaf/stem ratio than alfalfa grown under 16-hour photoperiods. The enhanced growth during long days often dilutes CP in herbage causing CP concentrations to be lowered.
Soil Nutrients
Nitrogen
Of all nutrients, nitrogen has the greatest impact on plant growth. Absorbed primarily as nitrate, nitrogen is rapidly taken up by roots. Most of the absorbed nitrogen is used in the synthesis of protein in a process involving reduction of nitrate to ammonium before incorporation into amino acids. Normally, nitrate concentration is low in plants, but environmental conditions that restrict growth, such as drought or mineral deficiencies, can result in accumulation of toxic levels of nitrate for livestock.
Application of nitrogen fertilizer usually has little or no effect on the extent of fiber digestion, but increases in rate of NDF digestion and intake by animals have been reported, especially if nitrogen concentration in the herbage was relatively low before nitrogen fertilization such as occurs in warm-season grasses (3). This response occurs because forage nitrogen is limiting for ruminal microbial growth under low-nitrogen situations. Other times, nitrogen fertilization may modify forage digestibility by altering the leaf/stem ratio.
Under favorable conditions application of nitrogen to grasses stimulates tiller development and increases leaf size and the period during which leaves stay green. Application of nitrogen fertilizer to grasses reduces soluble carbohydrate concentration. By rapidly stimulating growth, nitrogen fertilization tends to cause lower concentrations of other minerals in forage, primarily through dilution. Sulfur concentration of forage is more responsive to nitrogen fertilization than other minerals (3).
Phosphorus
The requirements for phosphorus are nearly equivalent for forage plants and the animals that consume them. Thus, if soil-available phosphorus is sufficient for vigorous growth of forage, phosphorus concentration in the forage should meet animal requirements. Where severe deficiencies occur, animal symptoms include poor intake and performance, breeding disorders, osteophagia (bone chewing), and rickets. Otherwise, concentrations of soil phosphorus have little effect on intake or DM digestibility (20). Because of the positive influence of phosphorus on legume proportion in mixed forage stands, phosphorus fertilization of pastures can indirectly increase animal performance.
Potassium
In contrast to phosphorus, potassium is required in substantially higher amounts by plants than by animals and limitations in soil potassium may limit yield, but are unlikely to have negative effects on animal performance. Potassium concentration has shown little effect on voluntary intake or DM digestibility (20). More commonly, excess soil potassium interferes with the uptake of calcium, magnesium, and sodium in grazing animals, which can induce grass tetany, a potentially fatal metabolic disorder.
Calcium
The concentration of calcium in forages depends on the amount of exchangeable calcium in the soil and also is influenced by soil nitrogen and phosphorus. Low concentrations of calcium in the blood of lactating animals cause parturient paresis (milk fever), a disease that is most prevalent when the prepartum diet is high in calcium, and it is brought on by the inability of animals to respond rapidly enough to the increased calcium demands of lactation. The ratio of calcium to phosphorus in the total feed is of some concern, as high calcium/phosphorus ratios have been implicated in a variety of disorders including high incidence of parturient paresis, poor breeding performance, and suboptimal feed conversion efficiency. The calcium/phosphorus ratio in alfalfa may be as high as 8:1, much greater than the recommended ratio of 2:1 (3).
Sulfur
Deficiencies of sulfur in soil, most common in highly leached, sandy soils, cause a marked decrease in true P concentration of forage, and can adversely impact ruminal microbial protein synthesis and fiber digestion. Bull (2) reported that cellulose digestion was enhanced with increases in sulfur concentration of corn silage up to .23% sulfur. Lignin concentration has been reduced by application of sulfur fertilizer to forage grasses in some studies, but has had inconsistent results in other studies and no effect in still other studies (3).
Plant Pests
Diseases
Diseased plants typically have lower digestibility and nonstructural carbohydrate concentrations than healthy plants, with variable differences in CP concentration. Moreover, diseases often cause leaf loss, which has an adverse affect on forage quality. Stem rust, caused by Puccinia graminis Pers., reduced digestibility of orchardgrass (7). Digestibility of smooth bromegrass decreased .12 percentage units for each 1% increase in diseased area of leaves (9). Plant resistance to diseases achieved through plant breeding can improve forage yield and nutritive value in the presence of disease organisms. Plant disease resistance seems to have little influence on forage yield or quality when the pathogen is absent (3).
Insects
Hutchins et al. (13) categorized insect pests broadly as either leaf-mass consumers or assimilate removers. Leaf-mass consumers damage plants mainly by consuming leaves and young developing buds. Assimilate removers typically possess piercing-sucking mouthparts and extract plant juices or otherwise disrupt translocation functions of plants. The effects of both types of insects is often reflected in alterations to the leaf/stem ratio of plants.
Defoliating insects initially remove mostly leaf material, which slows subsequent stem development and maturation of plants while the leaf area is being reestablished. The result can be yield reductions with only minor effects on forage quality. Primary insect defoliators of alfalfa include the alfalfa weevil (Hypera postica Gyllenhal) and the Egyptian alfalfa weevil (H. brunneipennis Boheman). Larvae of both species feed in stem terminals, where they skeletonize leaf tissue. Weevil feeding has only a moderate impact on alfalfa CP concentration and digestibility.
The second category of insects, assimilate removers, typically injure plants by extracting plant juices or otherwise disrupting translocation in plants. Aphids may have hormones associated with their saliva that change photosynthate translocation pattern within plants to their benefit. The potato leafhopper (Empoasca fabae Harris) is probably the most destructive assimilate remover of alfalfa in the USA. The most pronounced effect is stunting of alfalfa and reduced forage yield. The herbage produced has a higher leaf/stem ratio than that from noninfected alfalfa and a delayed rate of maturation. Hutchins et al. (14) found digestibility of alfalfa stems and leaves from stunted plants to be slightly higher than control plants that had not undergone potato leafhopper injury. Additionally, CP concentration of leaves was depressed while that in the stems was increased by potato leafhopper feeding. The overall effect on forage quality was small relative to effect of the insect damage on alfalfa yield.
Weeds
Some invading weeds reduce forage quality, whereas others, depending upon the species and maturity, may have little effect. Toxic or unpalatable weeds reduce animal performance if present in sufficient quantity to reduce the overall acceptability of the animal diet (3). Despite these negative effects, many weeds are comparable to forage species in chemical composition and quality. Marten and Andersen (19) and Temme et al. (27) found that redroot pigweed (Amaranthus retroflexus L.), common lambsquarters (Chenopodium album L.), and common ragweed (Ambrosia artemisiifolia L.) had chemical traits and digestibility similar to those of alfalfa in Minnesota and Wisconsin. Likewise, dandelion (Taraxacum officinale Weber), white cockle (Lychnis alba Mill.), and immature Jerusalem artichoke (Helianthus tuberosus L.) have forage quality equal or superior to many high quality forage species. Moreover, quackgrass [Agropyron repens (L.) P. Beauv.] forage yield and quality are similar to those of commercial cultivars of cool-season grasses adapted to the North Central Region of the USA (3).
Conversely, giant foxtail (Setaria faberii Herrm.), Pennsylvania smartweed (Polygonum pennsylvanicum L.), yellow foxtail [Setaria glauca (L.) P. Beauv.], barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.], and shepherd's purse [Capsella bursa-pastoris (L.) Medic], among other weed species, have relatively poor forage quality (1, 19, 27). Dutt et al. (6) reported that animal intake and digestibility of alfalfa were reduced by infestation with yellow rocket (Barbarea vulgaris R. Br.). Cords (4) found a negative correlation between CP concentration of alfalfa-weed hay and presence of the mature winter-annuals flixweed [Descurainia sophia (L.) Webb], downy bromegrass (Bromus tectorum L.), and wild barley (Hordeum leporinum Linl.) in Nevada. The rate of decline in digestibility with time was generally greater for the weeds than for alfalfa.
References