Introduction
Because fermentation is based on microbial metabolism it is important to understand the role of the microorganisms involved in the process. Lactic acid producing bacteria, enterobacteria, clostridia, and yeast are the primary organisms of interest during ensiling. Naturally occurring LAB are responsible for converting soluble sugars to acids which lowers the pH and preserves the silage. Enterobacteria can compete with LAB for sugars and can produce detrimental endotoxins that could reduce animal performance. Eliminating oxygen and acidification will inhibit their growth. Yeasts and molds grow well in the presence of oxygen. Yeast can also compete for fermentable substrates and can use acids. It is well established that yeasts are primarily responsible for aerobic spoilage of silages. Molds can be detrimental because they can produce mycotoxins which have negative effects on animal health and performance. Clostridia are organisms that thrive under moist conditions and degrade sugars and proteins. They can be controlled by wilting forage above 30% DM prior to ensiling. Fermentation pathways from yeast, molds, and clostridia are extremely inefficient and can result in large losses of DM and energy.
Microbial Inoculation
Common LAB used in silage inoculants are:
Inoculants may contain one or more of these bacterial strains which have been selected from the natural environment for their ability to rapidly lower the pH in silage. Multiple organisms are used for various reasons. For example, pediococci are sometimes used because some are more active during the early stages of ensiling than lactobacilli. In addition, some pediococci are more active than some lactobacilli in cool conditions. Although many inoculants appear to have the same organism, users should be aware that products from different suppliers may vary tremendously because of strain differences (e.g., there are many different strains of Lactobacillus plantarum). Recently, Propionibacteria have been included in some silage additives in hopes of improving aerobic stability of silages. These bacteria convert lactate and glucose to acetate and propionate. Higher levels of propionate (and to some extent acetate) would theoretically inhibit spoilage fungi. Effects of adding Propionibacteria to silage from our lab (Kreck and Kung, University of Delaware unpublished data) and from the USDA Forage Research Center (R. E. Muck, personal communication) has not been encouraging. Data from our lab suggests that most strains of Propionibacteria are acid intolerant and exhibit poor growth when the pH is below 4.4. Thus, more acid tolerant strains of Propionibacteria must be identified for use in cereal silages where pH is often below 4.0.
In North America, some have suggested that inoculants supply 100,000 (105) organisms per gm of forage for maximum effectiveness and it is probably uneconomical to add 106 organisms per gram of forage as is done in Europe where silages are significantly wetter. However, users should understand that the efficacious organism(s) are needed.
Microbial inoculants are sold in a dry (powder or granular) or liquid form. Use of chlorinated water may be detrimental to the bacteria. If chlorine content is in question, the addition of 1 cup of milk solids to about 200 liters of water will neutralize the chlorine. Application can be with a simple watering can by weighing the incoming forage load and adjusting the application based on the average unloading time. A better method is to use a metered liquid sprayer to evenly disperse the inoculant on the forage. Most manufacturers suggest discarding unused liquids after a 24 to 48 h period. If forage is hauled long distances prior to ensiling (as in some places in southern California) application would be better if it were done at harvest (rather than several hours later at the point of ensiling). Proper distribution cannot be overlooked and is important for the inoculant to be effective. Throwing a cup of dry inoculant into a wagon load of forage and hoping for even distribution is not acceptable.
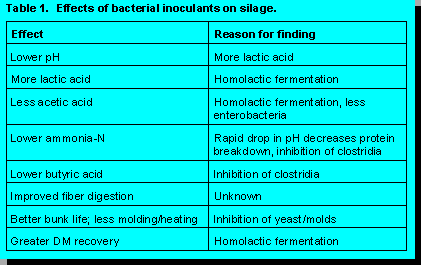
Bacterial inoculants have improved fermentation and animal performance in cereal silage (5), grass silages (3) and alfalfa silage (4). When compared to untreated silages, inoculated silages usually (but not always) are lower in pH (due to higher lactic acid), acetic acid, butyric acid and ammonia-N (Table 1). However, changes in fermentation end-products, digestibility, or intake are meaningless unless accompanied by one or more of the following:
 Recently, Muck (6) summarized
studies with inoculants from 1985 to 1992. Recovery of DM was improved in about 60%
of the studies, intake and gain were improved in about 25% of the studies, and milk
production and feed efficiency were improved in about 40% of the studies.
Recently, Muck (6) summarized
studies with inoculants from 1985 to 1992. Recovery of DM was improved in about 60%
of the studies, intake and gain were improved in about 25% of the studies, and milk
production and feed efficiency were improved in about 40% of the studies.
Because the cost of inoculation is fairly low (typically $.50 to 1.50 (US dollars) to treat a ton of forage) small benefits can yield good pay backs. However, the producer should not be fooled by the low cost and only use an inoculant when deemed necessary and when proven effective. Inoculants are not a substitute for good management and cannot overcome poor forage quality. In addition, if fermentable substrates are limiting, they may not be effective. One way to provide adequate fermentable substrates may be to use cell wall degrading enzymes with an inoculant.
Enzymes as Silage Additives
Use of NPN Compounds
Addition of anhydrous ammonia or water-ammonia mixes initially buffers the plant material. For example, corn forage may have a pH of 5.9 (slightly acidic), but treated corn forage will have a pH of about 8.5 to 9.0 (very alkaline). We have observed that ammonia treatment causes an initial delay followed by stimulation in growth of bacteria which make lactic acid and pickle the forage. When fermentation in the silo is complete, corn silage treated with anhydrous ammonia usually is .1 to .2 units higher in pH, contains .5 to 1.5% (DMB) more lactic acid, .5 to 1.5% more acetic acid, and less residual water soluble carbohydrates. Forages treated with ammonia have also been shown to be higher in insoluble N and true protein (both of which are beneficial) primarily because ammonia reduces plant proteolysis. Ammonia has been suggested to be anti-fungal in nature and results in improved aerobic stability (reduced molding and heating) during storage and feedout. However, recent data from our lab would suggest that improved aerobic stability is due to an increase in acetic acid and decrease in residual water soluble carbohydrates after ensiling, not due to a direct fungicidal effect from ammonia. Regardless of what the mechanism is, bunk life is improved.
Ammonia can be added at the chopper, blower, bagger, or bunk. Mixed ammonia solutions are bulkier than anhydrous ammonia, but retention of ammonia is usually greater. In addition, molasses (to improve palatability and fermentation) and minerals can be added in these solutions. Some ammonia will be lost (between 10 and 30%) and losses will be greater if ammonia is not applied properly and if forage becomes too dry. Ammonia should be applied to the forage before it contacts the blower to minimize losses. Ammonia should be added at the end nearest the cutter in a chopper with an auger system. If no auger is used, ammonia can be added behind the cutter prior to entering the blower. Ammonia can also be spiked into bunks between loads and it will disperse into the mass.
Application of anhydrous ammonia should be at approximately 7.9 kg of N per 1,000 kg of forage DM. Excess ammonia may result in poor fermentation (because of a prolonged buffering effect) and animal performance. Adding 3.2 kg of ammonia per tonne of 35% DM corn silage will increase the CP from about 8% to 12.5% (DM basis). Using the Cold- flo method is the simplest way to add ammonia to silage. Gaseous ammonia is super cooled in a converter box and about 80 to 85% becomes liquid. Anhydrous ammonia should not be added to corn forage if the DM content is above 40 to 42% because fermentation is restricted in drier material and binding of ammonia will be less; thus normal fermentation may be disrupted. In instances where forage DM is above 40 to 42%, water-ammonia mixes or molasses-ammonia mixes should be used. Application for molasses-ammonia mixes should be as recommended by the manufacturer.
Ammonia is a hazardous gas and should be handled with care. Eye protection should be worn when making connections to pressurized tanks. Water should be available at all times. Ammonia is also corrosive to zinc, copper, and brass; therefore, storage of ammonia-treated forage in zinc coated steel silos is not recommended. Problems with hyper-irritability (bovine bonkers syndrome) in cattle fed ammoniated forages has not been observed in cattle fed ammoniated corn forages. Addition of ammonia to corn silage has no effect on nitrate levels in corn silage.
There has been recent interest in adding anhydrous ammonia to alfalfa silage, primarily to improve aerobic stability. Certain precautions must be considered for this application. First, alfalfa silage already contains excess amounts of rumen degradable protein and added ammonia will compound this problem. Secondly, there is some research that shows that when alfalfa is on the wet side (less than 30 to 33% DM) ammonia can cause an undesirable clostridial fermentation leading to high levels of butyric acid and protein degradation in the silo. If you do treat alfalfa silage with ammonia, be sure to discount the added nitrogen when balancing the diet. At the time of writing this article, this author would not recommend widespread use of ammonia on alfalfa silage until more research is conducted.
Propionic Acid as a Silage Additive
Summary
References