Introduction
As highly fermentable carbohydrate is introduced to the diet, ruminal VFA production actually increases as pH starts to decrease. A critical threshold for ruminal pH to achieve acute acidosis is <5.0 and subclinical acidosis, <5.5 (27). Ruminal changes initiate several systemic changes. Increased organic acid, particularly lactic acid, and a reduction in pH results in decreased ruminal motility, stasis, rumenitis, and hyperkeratosis (10, 33). In many dairy operations, the challenge is not with acute, but sub-clinical acidosis. Daily ruminal pH below certain thresholds (5.0) for given periods of time predispose cattle to low grade, subclinical acidosis with symptoms including: erratic appetite, loss of body weight, diarrhea, and lameness.
Laminitis is a multi-factorial metabolic disorder that occurs in the acute, sub-clinical, and chronic forms (4, 16, 21, 38). However, the sub-clinical state often goes unnoticed, as it can result in significant intangible losses. Manifestations of sub-clinical laminitis are sole hemorrhages and yellowish discoloration of the sole (4, 39). Other indicators include: double soles, heel erosion, dorsal wall concavity, and ridging of the dorsal wall, (39). The critical link between nutrition, acidosis, and laminitis appears to be associated with altered hemodynamics of the peripheral microvasculature (6).
A critical challenge for the dairymen is to identify occurrence of both sub-clinical acidosis and laminitis and make appropriate adjustments in feeding and husbandry management practices.
The objective of this manuscript is to 1) identify nutritional factors which predispose cows to acidosis, and 2) characterize the link between acidosis and laminitis.
Acidosis
The initial nutritional factor thought be associated with the development of laminitis is commonly a result of acidosis. Acidosis is caused by the ingestion of greater than "normal" quantities of ruminally fermentable carbohydrates. The clinical symptoms of carbohydrate overload are manifested in a variety of ways and degrees, depending upon the types and amounts of carbohydrates consumed. As a result of carbohydrate overloads, unphysiological limits of acids are produced with a concomitant reduction of pH, which overwhelms the ruminal and host (systemic metabolism) system. The cascade effects of acidosis originating from the initial ingestion of carbohydrate depends upon the intensity and duration of the insult. Most critical is the pH threshold. This not only relates to microbial growth rates and shifts in ruminal populations, but significantly influences the systemic metabolic state and ability to catabolize and/or excrete certain metabolites.
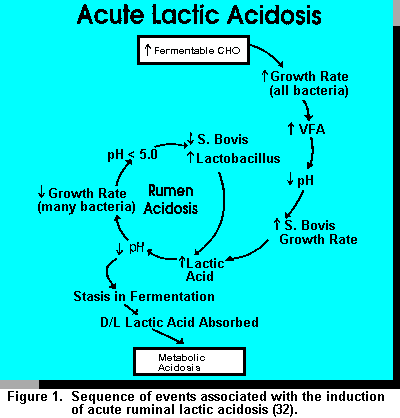
Figure 1 illustrates the developmental process of lactic acidosis to a systemic/metabolic state. Initially, as increased fermentable carbohydrates are fed, there is an increased growth rate of all bacteria. These events result in an increased level of VFA produced with a concomitant decrease in ruminal pH. As ruminal pH declines further, the growth rate of S. bovis increases relative to other bacteria. Lactate dehydrogenase is stimulated and there is a net change in S. bovis metabolism from acetate and formate to lactic acid production (45). The accumulation of lactic acid exceeds the ruminal buffering capacity. As ruminal pH declines further (<4.7), growth rate of S. bovis decreases, which creates a niche for lactobacilli to develop, producing greater amounts of lactic acid (45). If the carbohydrate insult continues to persist, the spiralling effect also persists and irreversible metabolic acidosis in inevitable.
Options to Reduce Lactic Acid in the Rumen. A generally accepted concept for ruminal lactic acid accumulation is that lactate producers outnumber utilizing bacteria (44). Critical to this imbalance is pH optimization for growth. Russell et al. (45) demonstrated that the growth rate of S. bovis (major lactic acid producer) falls off dramatically between pH 5.3 and 5.1. Also, Megasphera elsdenii has a significantly reduced growth rate from pH 6 to 5.5. This differential results in significant accumulation of lactic acid when readily fermentable carbohydrate is provided. When ruminal pH is maintained above 5.5, a balance and equilibrium exists between producers and utilizers, such that lactic acid does not accumulate in the rumen.
Rumen contents become hypertonic in relationship to plasma in acidotic animals. (11, 17). A key regulatory method associated with the maintenance of systemic pH is the bicarbonate buffering system, if entry of lactic acid into body fluids is not overwhelming. This mechanism is usually able to maintain pH within critical limits until the acidotic process has run its course.
Sub-Clinical Acidosis
Acute acidosis presents specific signs and symptoms which, if caught in time, can be treated directly. Clinical symptoms of sub-clinical acidosis are insidious and considerably less overt. Often times sub-clinical acidosis is dismissed for other problems, such as poor forage quality, poor bunk management, etc. and is not addressed. Therefore, it can be a tremendous economic sink, such that it drains productive efficiency potential from dairy herds. The major clinical manifestation of sub-clinical acidosis is reduced and/or cyclic feed intake. Other associated signs include: decreased efficiency of milk production, reduced fat test, poor body condition despite adequate energy intake, high culling rate, unexplained diarrhea, and episodes of laminitis.
Sub-clinical acidosis is a consequence of maximizing energy intake. In order to do so, we must follow the fine line of providing appropriate levels of physical versus chemical dietary components. Often times, sub-clinical acidosis is found in well-managed, high producing herds.
Rumen pH as a Diagnostic Tool to Detect Sub-Clinical Acidosis. Sub-clinical acidosis is a temporarily altered rumen state which causes some aberration in fermentation patterns and decreased rumen pH, however, intensity and duration are not adequate to cause immediate overt clinical signs. The challenge is that other disease processes also can cause symptoms listed previously. Nutritional alterations (i.e., adequate effective fiber, altering forage: concentrate rations, feeding strategies, etc.) influence ruminal pH which can affect the occurrence of sub-clinical acidosis. The only determinative diagnostic test of sub-clinical acidosis is ruminal pH.
Stomach tubing to decrease rumen pH is plagued with false interpretation because of saliva contamination. Rumen cannulation is the preferred method of obtaining representative samples of rumen fluid, although this has traditionally been used for research purposes.
Norlund and Garrett (37) discuss rumenocentesis or percutaneous needle aspiration as a means of collecting rumen fluid for diagnosis of sub-clinical acidosis in dairy herds. Our laboratory conducted a study to evaluate rumenocentesis. There were no significant differences between multi-sample composites and one sample ventral rumen aspiration. Samples taken at 1600 hours by rumenocentesis were .1 to .2 pH units higher than the average of the composite and single sample aspiration. Garrett et al. (14) compared needle rumenocentesis versus cannula collections and found needle probes were approximately .35 units lower than sample obtained via rumen cannula. The following equation related cannula pH and needle probing: cannula pH = 3.257 + .506 (needle pH), r = .73 P > .01.
In order to reach nadir when feeding a TMR once daily, rumen samples should be taken five to eight hours post-feeding. These recommendations coincide with those of Norlund and Garret (36) for feeding a TMR. Animals fed forage and concentrate separately should be sampled two to five hours post-concentrate feeding (36). Animal selection should be from high risk groups: in the first 60d post-partum. Since considerable animal variation is inherent, a minimum of 10 animals per group should be considered. Norlund and Garrett (37) indicated that if more than 30% of the animals within the particular sub-group have pH of 5.5 or less, the group should be considered abnormal, between 5.6 and 5.8: marginal, above 5.8: normal. This type of diagnostic tool should be used in conjunction with other factors such as ration evaluation, evaluation of management practices, and identification of health problems on a herd wide basis.
Risk of Acidosis
Although there are guidelines, high producing cows consuming large quantities of grain (55 to 60% of DM) will have a tendency toward lower ruminal pH's during the day. The critical question yet to be addressed is how low can pH go, and for how long before negative effects will be demonstrated? There are different physiological occurrences that normally take place in a cow's transition during the lactation cycle which predispose her to higher risks of acidosis.
During the transition period and through 50 days postpartum, management of the cow mediated events play an important role in the development of acidosis. Interpretation in the "normal ecological balance" within the rumen can ultimately play a role in predisposing the animal to sub-acute acidosis. When intake is reduced, energy metabolism of the rumen microorganisms as well as the host system is interpreted. Intake is controlled by a balance of physical and chemostatic mechanisms. The challenge is to ensure that both mechanisms are working in synchrony, such that one does not overpower the other (i.e., too high grain, and/or fermentable carbohydrate versus too much forage).
Although there are several factors (i.e., heat, cold facilities, management, diet composition, etc.) that influence intake, managers ultimately must anticipate and compensate for intake challenges associated with normal daily practices on a given farm.
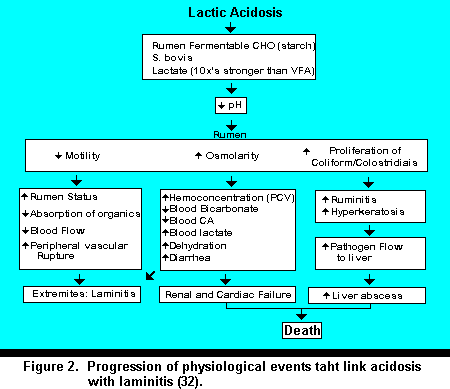
The Implications of Acidosis on the Development of Laminitis. The progression from acidosis to laminitis is associated with several systemic phenomena. Figure 2 outlines several aspects associated with the development of laminitis. A very strategic and critical apex in this entire cascade process is the reduction in ruminal pH. The effects of pH on the pathogenesis of the rumen, liver, and GI tract ultimately affect hemodynamics and predispose animals to laminitis. The onset of acute acidosis presents hemodynamic insults that predispose animals to severe, but short episodes of acute laminitis. Several severe episodes of short duration or low insidious levels of acid can create a situation which predispose the animal to vascular destruction, which can ultimately result in a low grade, but irreversible laminitic process.
Laminitis
 Definition and Incidence. The scientific name
for laminitis is "pododermatitis aseptic diffusa". Translated it means an aseptic inflammation of
the dermal layers inside the foot. Surveys (9, 12, 39, 41, 48, 49) indicate mean lameness ranges
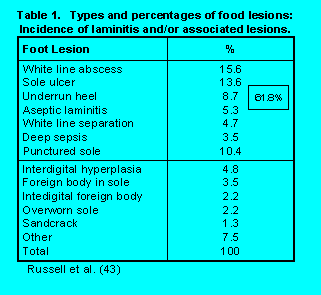
from 5.5 to 30%. Within surveys, herds ranged from 0 to 55% lameness. Table 1 illustrates the
types and percentages of foot lesions found in the largest survey (43). Of the total spectrum of
lesions identified, approximately 62% of these manifested could be associated with laminitis in
some form, suggesting it's importance as a cause of lameness.
Definition and Incidence. The scientific name
for laminitis is "pododermatitis aseptic diffusa". Translated it means an aseptic inflammation of
the dermal layers inside the foot. Surveys (9, 12, 39, 41, 48, 49) indicate mean lameness ranges
from 5.5 to 30%. Within surveys, herds ranged from 0 to 55% lameness. Table 1 illustrates the
types and percentages of foot lesions found in the largest survey (43). Of the total spectrum of
lesions identified, approximately 62% of these manifested could be associated with laminitis in
some form, suggesting it's importance as a cause of lameness.Causes of Laminitis. Often times laminitis is referred to as a syndrome. It has a multi-factored etiology, and it is thought to be associated with a spectrum of largely interdependent factors (26, 38). Although, nutritional management influences the development of laminitis, metabolic and digestive disorders can also predispose animals to laminitis. Hormonal changes associated with parturition and other phases of the animals lactation cycle can have an impact on certain physiological changes. Infectious diseases such as mastitis, metritis, foot rot, etc. can impose specific endotoxic insults (22). Environmental aspects such as hard stall surfaces, lack of bedding, or little use of bedding, lack of excessive exercise, or excessive exercise on undesirable surfaces can predispose animals to mechanical damage (4). Other factors such as body condition, body weight, and feet and leg structure can unnaturally increase the weight load and stress on feet exacerbating internal mechanical damage associated with laminitis.
Mechanisms of Laminitis Development. The mechanistic phases of laminitic development can be best described as alternating stages of disturbances relating to metabolic and subsequent mechanical breakdown of the internal foot structure. Review articles and studies have dealt with specific phases (5, 26, 38). The process can be segmented into various phases.
 The initial activation phase of laminitis is associated with a systemic metabolic insult resulting in
a decreased ruminal, and subsequently systemic pH. The reduction in systemic pH activates a
vaso-active mechanism which increases digital pulse and total blood flow. Depending upon the
insult which initiates the process, endotoxins and histamine can be released which create
increased vascular constriction and dilation. This in turn causes the development of several
unphysiological arterial-venous shunts, further increasing blood pressure. The increased blood
pressure cause a seepage through vessel walls, which ultimately are damaged.
The initial activation phase of laminitis is associated with a systemic metabolic insult resulting in
a decreased ruminal, and subsequently systemic pH. The reduction in systemic pH activates a
vaso-active mechanism which increases digital pulse and total blood flow. Depending upon the
insult which initiates the process, endotoxins and histamine can be released which create
increased vascular constriction and dilation. This in turn causes the development of several
unphysiological arterial-venous shunts, further increasing blood pressure. The increased blood
pressure cause a seepage through vessel walls, which ultimately are damaged.
Next, there is mechanical damage associated with the vascular system. Once vascular edema has occurred, ischemia (local anemia) results in hyperemia of the local internal digital tissue causing tissue hypoxia resulting in less nutrients and 02 reaching the epidermal cells. Ischemia itself can further cause an increase in AV shunting. Trauma, stress, as well as certain hormonal and/or chemical releasing actions can increase AV shunting. As a result of previous events, increased blood pressure further increases vascular seepage in the lower part of the digit as well as edema and ischemia. This cycle continues as long as the initial insult remains (32).
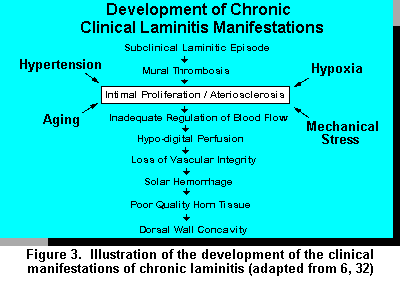
The mechanical damage associated with micro-vasculature results in less nutrients reaching epidermal cells, the stratum germinativum in the epidermis breaks down. These events ultimately cause corium degeneration and breakdown of the laminar region associated with the dermal-epidermal junction. As the epidermal junction is broken down, separation of tissue layers (S. germinativum and S. corium) occur. As the laminae layers separate, the pedal bone takes on a different configuration in relationship to its position in the corium and dorsal wall. As the bone rotates and/or shifts in position, it causes a compression of the soft tissue between the bone and sole, which is extremely susceptible to damage. This results in hemorrhage, thrombosis, and ultimately further enhances edema and ischemia resulting in a necrotic area within the solar region of the foot. Ultimately a variety of processes can happen as a result of the scar tissue intervention including double sole phenomena, sole hemorrhages (red blood patches), bruises, diffuse lesions, solar pulp, etc. (Figure 3).
Having characterized the mechanistic development of laminitis, one can see the physiological events that take place in relation to anatomical changes and ultimately the degree of clinical manifestation of the various forms of laminitis.
Sub-Clinical Laminitis.
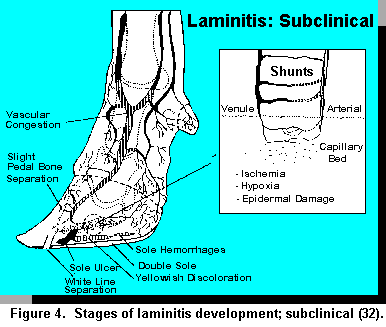 This particular form of laminitis can be a long, slow, insidious process dependent upon the
persistency of low grade insults. The horn becomes physically softer during this period with the
yellowish coloration of the sole caused by serum seepage from the vessels into the solar corium.
Hemorrhagic stains are manifested in the solar area, particularly in the white line regions, the apex
of the sole, and sole/heel junction. Internally, ischemia, hypoxia, and epidermal damage are key
aspects associated with this stage. AV shunting becomes an increasing occurrence. White line
separations and dorsal wall ridges are often observed and a slight tilting to definite rotation of the
pedal bone are characteristic internal manifestations. Sometimes the soles of affected feet are soft
and warm, with no visible lesions. This may be associated with integral hemorrhagic areas. A
possible theory on the development of heel cracks and double soles may be that a hemorrhagic
area, once walled off, become a perfect environment for bacteria (anaerobic) to grow. As gas and
exudate develop, they are forced by pressure to points of least resistance, which often is the
sole/hock bulb junction (32). Figure 4 depicts the anatomical and physiological aspects associated
with development of subclinical laminitis.
This particular form of laminitis can be a long, slow, insidious process dependent upon the
persistency of low grade insults. The horn becomes physically softer during this period with the
yellowish coloration of the sole caused by serum seepage from the vessels into the solar corium.
Hemorrhagic stains are manifested in the solar area, particularly in the white line regions, the apex
of the sole, and sole/heel junction. Internally, ischemia, hypoxia, and epidermal damage are key
aspects associated with this stage. AV shunting becomes an increasing occurrence. White line
separations and dorsal wall ridges are often observed and a slight tilting to definite rotation of the
pedal bone are characteristic internal manifestations. Sometimes the soles of affected feet are soft
and warm, with no visible lesions. This may be associated with integral hemorrhagic areas. A
possible theory on the development of heel cracks and double soles may be that a hemorrhagic
area, once walled off, become a perfect environment for bacteria (anaerobic) to grow. As gas and
exudate develop, they are forced by pressure to points of least resistance, which often is the
sole/hock bulb junction (32). Figure 4 depicts the anatomical and physiological aspects associated
with development of subclinical laminitis.
Vaso-Active Substances. Destruction of the normal hemodynamic process has been identified as a major etiological factor associated with the development of laminitis (5, 22). Vaso-active substances play a critical role in affecting hemodynamics. Histamine has long been identified as a potent vaso-dilator and arterial constrictor (7) and naturally found in tissues and blood. The hemodynamics associated with the circulatory effects of the histamine on laminitis coincide with mode of action.
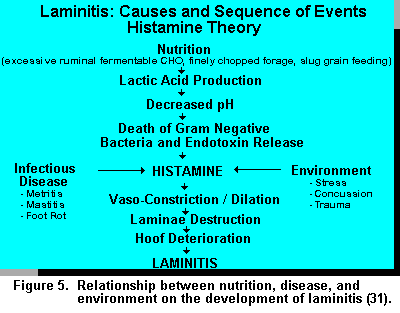
The involvement of histamine and endotoxins fit well with the nutritional theory of laminitis development (Figure 5). However, histamine release can be caused by a variety of factors other than nutritional origin such as: environmental stress, concussion, trauma associated with concrete floors, overcrowding, infectious diseases, etc., causing tissue breakdown. This process accentuates the dynamics of the etiology of laminitis particularly since many of these events occur during the first 50 days postpartum (42).
Nutrition and Laminitis
Although it has been stated that the etiology of laminitis is multi-factorial, nutrition has received considerable recognition as the cause. A major challenge regarding nutrition is a lack of information to specify threshold levels of carbohydrate (both structural and non-structural) that initiates nutritional insult (i.e., acidosis). Carbohydrates comprise approximately 70 to 80% of the dairy cow ration, therefore, the level and availability in various rations can have a significant impact on rumen metabolism.
The amount of feed necessary to induce ruminal acidosis depends on the type of carbohydrate processing, adaptation period, nutritional status of the animal, and frequency of which the carbohydrate (starch) is fed (13, 27, 29, 30). Nocek and Tamminga (35) show rates and extents of ruminal digestion of various feedstuffs. Barley, wheat flour, oats, and steam flaked corn all have ruminal starch availabilities greater than 85%. However, processing (grounding, steam-flaking, chemical treatment) can have a major influence on availability (35, 47). Ruminal adaption is critical in determining the amount of carbohydrate that will induce acidosis (27).
Relatively few studies have evaluated the direct influence of carbohydrate source on the incidence of laminitis. Manson and Leaver (25) offered a 60:40 or 40:60 ratio of concentrate to silage to cows during weeks 3 to 26 of lactation. The 60:40 TMR was restricted to yield the same metabolizable energy intake between treatments. Hooves on half the animals for each treatment were trimmed prior to the experiment, the other half remained untrimmed. The 60:40 diet increased locomotion scores, number and duration of clinical lamenesses, decreased hoof hardness, increased milk protein, and had no effect on milk yield when compared to the animals fed the higher forage diet. Trimming hooves reduced the number and duration of clinical lamenesses. In addition, hoof growth was significantly increased by trimming. The low concentrate diet had 8 lame cows, 3.3 wk in duration, whereas the high concentrate diet had 11 lamenesses, 3.9 wk in duration.
Since intake was restricted on the 60:40 diet and grain fed in a TMR, the major difference between dietary treatments was concentrate intake (9.1 versus 5.8 kg concentrate or 5.7 and 1.7 kg of barley). Cows fed high concentrate produced 1.2 kg more milk, .15 percentage units more protein and produced .16 kg milk/kg DM more than cows fed low concentrate. This study, therefore, demonstrates the effect of carbohydrate level and availability on lameness, but also shows that increased non-structural carbohydrate (NSC) levels enhances production performance.
In another study, Manson and Leaver (23) compared 7 versus 11 kg of concentrate/d from 3 to 22 wk into lactation. Cows fed high concentrates had more lameness, of greater severity and duration, with sole lesions being the major problem. However, cows fed more grain produced 3.2 kg more milk/day with .06 percentage units higher milk protein. Other researchers (19, 26) have shown a relationship between feeding high carbohydrate diets, feeding frequency, and severity of digital lesions.
We can control the incidence of laminitis through nutrition i.e: feeding high fiber diets, and use of good feeding and management practices. However, most dairy producers are in the business to make a profit. Increasing concentrate intake to a certain threshold maximizes milk production and milk components. In doing so, dairy managers walk the fine line between maximizing profit and dealing with sick and lame animals.
 The way feeds are fed can have a significant impact on the stability of ruminal pH. As the amount
of concentrate goes up in the diet and or the diet increases in fermentable carbohydrates, saliva
production and time of rumination decreases which decreases ruminal pH. Kaufmann et al (18)
showed that feeding frequency of concentrates has a significant impact on containing a stable
ruminal pH. Nocek (30) identified that feeding the same ingredients and forages in different
strategic fashions during the day can influence the time ruminal pH remains below a given
threshold. .
The way feeds are fed can have a significant impact on the stability of ruminal pH. As the amount
of concentrate goes up in the diet and or the diet increases in fermentable carbohydrates, saliva
production and time of rumination decreases which decreases ruminal pH. Kaufmann et al (18)
showed that feeding frequency of concentrates has a significant impact on containing a stable
ruminal pH. Nocek (30) identified that feeding the same ingredients and forages in different
strategic fashions during the day can influence the time ruminal pH remains below a given
threshold. .
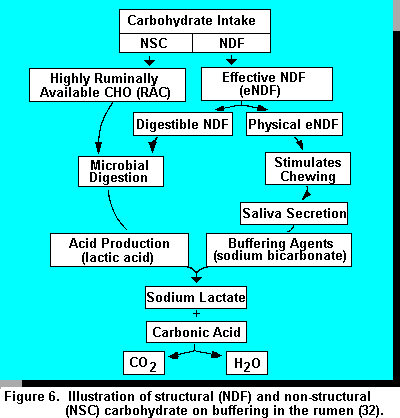
The influence of carbohydrate on rumen pH is the critical link between nutrition, acidosis, and laminitis. Although excessive levels of ruminally available carbohydrate are blamed for increased acid production and overwhelming the bicarbonate buffering systems, lack of effective fiber can significantly influence saliva production and ruminal pH (Figure 6). Effective fiber stimulates chewing, which stimulates saliva secretion. Buffers in saliva neutralize acids produced by ruminal fermentation. Therefore, ruminal pH is a balance between acid production from carbohydrate substrates and saliva production (buffering action). Substantially more information is needed to quantify physically effective (pe) NDF. Just because a certain proportion of particles are greater than 2.54 cm, does not render it effective. It may be digested or contribute to acid production, especially improperly fermented, wet forages with high NSC and lactic acid levels..
The relationship between ration NSC, rumen available starch, NDF, and eNDF is critical in maintaining proper rumen function. Poore et al. (40) fed diets similar in NDF (30%) in a factorial study where sorghum was either dry rolled or steam flaked and wheat straw was substituted for alfalfa hay. Chopped wheat straw substituted for alfalfa hay did not affect milk yield or composition. Increasing starch degradability increased milk yield and protein regardless of fiber source. These researchers suggest that a ratio of forage NDF to ruminally degradable starch should be maintained (about 1:1) to avoid depression in fiber digestion and maintain normal rumen function. Nocek and Russell (34) showed milk yield to be maximized when the NSC to NDF ratio was between .9 and 1.2..
The addition of hay to diets does less to enhance the effective fiber of the diet than increasing the particle size of silage (3). Although small amounts of hay added to the diet increase intake compared to increasing silage particle length, it did not appear to affect milk production or composition in diets considered low in effective fiber (3). No reference was made to rumen pH thresholds in these studies..
Guidelines for Inclusion of Different Carbohydrate Fractions in Rations of Lactating Dairy Cows
Based on studies with lactating dairy cows producing greater than 35 to 40 kg of milk (29, 34) general guidelines for different carbohydrate fraction concentrations can be considered (% of total ration DM):
When considering rumen degradability parameters, the following general guidelines can be
considered:
These guidelines should only be considered after total ration energy (NEL) requirements (NRC) are met for the specific animals in question.
Other guidelines regarding physical form and rations of structural to non-structural carbohydrate
include:
Factors such as the amount and type of grain, grain processing, forage type, quality, levels, etc. influence intake patterns, energy metabolism, and sub-clinical acidosis. Rate and extent of digestion and amount of starch intake modifies the severity of acidosis. Grain mixes containing finely ground or highly processed cereal grains (i.e., corn, wheat, barley) have the fastest rates of ruminal starch digestion (35). It is critical that the grain portion of the diet complement the forage type and quality and contain ingredients with various levels of rumen carbohydrate availability. Both low roughage levels and reduced particle size exacerbate acidosis during the transition period.
Protein in the Ration. Although not covered extensively in this review, the amount of ration protein has been shown to influence the incidence of laminitis. Manson and Leaver (24) compared feeding a 16.1 versus 19.8% crude protein diet. The high protein diet significantly increased locomotion scores, number, and duration of clinical lamenesses for dairy cattle between 3 and 26 wk post-partum. Bargari et al. (1) studied the influence of feeding 15.3 versus 18% crude protein on farms with "normal" calves and those affected with laminitis. Blood urea nitrogen levels on affected farms were elevated in both normal and affected calves in affected herds. Total protein globulin and packed cell volume were elevated in affected animals on affected farms. Therefore, high levels of rumen degradable protein have been identified as a factor of increased lameness and laminitis. Logue et al. (20) suggests that the inclusion of animal protein (blood meal, fishmeal, and meat and bone meal) may serve a preventative function compared to vegetable protein sources (soybean meal). However, this study showed no influence of protein source on lameness. There is little information available to identify what role protein may play in the development of lameness. Several postulations involve allergic histaminotic reactions to certain types of proteins (28) or a link between high protein supplementation and protein degradation end-products (2).
Environmental Factors Associated with Controlling Sub-Clinical Acidosis and Laminitis
Although carbohydrate nutrition has a direct link with acidosis and laminitis, other factors including housing, stage of lactation, and age have an influence on the predisposure of animals to sub-clinical acidosis and laminitis.
Separate First Calf Heifers into Specific Groups. Often times laminitis in first calf heifers is associated with changes in management such as introduction into groups of mature cows around calving and into stalls with inadequate bedding or poor design, such that ease of maneuvering in and out of the stall is difficult. There is a relationship between time spent standing, use of stalls, and the incidences of sole ulceration and laminitis in first calf heifers (15). Colan-Ainsworth et al. (8) showed that supplying increased bedding to stalls eliminated new cases of laminitis and sole ulceration in heifers.
Provide Adequate Exercise. Locomotion has a significant influence on the hemodynamics of the peripheral circulation of the foot. Too little exercise, i.e., standing, can cause sluggish blood flow, edema, and swelling. On the other hand, too much exercise and concussion on concrete floors, especially for heifers that have been accustomed to pasture, can cause trauma and mechanical damage with a greater incidence of sole ulceration (4).
Stall Comfort. First of all, adequate stall space must be provided to allow reclining and ruminating for approximately 12 to 14 hours per day. The dimensions of the stall must be proper for the size animal that is being housed, especially stall length, width, and lunge space. Bedding should be non-abrasive. Sand is an optimal stall surface with regard to providing cow comfort, traction, and lack of organic matter for bacterial growth that may predispose animals to mastitis. However, management of sand may be a challenge regarding burrowing and destruction to manure systems. In addition, sand must be free of small stones which can penetrate the solar horn. An earthen base with shredded tires covered with polyethylene sheets also works effectively in providing a cushioned base. However, this material must not be of an abrasive nature to allow scraping of hocks and knees as animals are rising and laying down. The use of sawdust (with wood chips) on these polyethylene surfaces can also be abrasive, causing hock lesions, etc.
Age & Stage of Lactation. In a survey conducted in the United Kingdom, Rowland et al. (42) showed that susceptibility to lameness increased with age, i.e., 10 year old cows were four times more likely to develop lameness than 3 yr olds. The most prevalent lesions were white line abscesses, sole ulcers, and under run heels. The incidences of these diseases with age were thought to be caused by cumulative factors (scar tissue, etc.). The same study showed that lameness was more common during the first 120 days postpartum, accounting for 51% of all cases that occurred. Solar ulcers were somewhat more common during the 30 days after calving than before calving, whereas, white line abscesses were more common toward the end of lactation.
Conclusions
Nutrition and laminitis are linked through imbalances in carbohydrate nutrition: overload of ruminally fermentable carbohydrate in conjunction with inadequate effective fiber. The subclinical phase of both disease processes is most costly and damaging because they are often dismissed for other problems, allowing the disease to progress to an irreversible chronic phase. Since the highest incidence of laminitis is detected during the first 30 to 40 days post-partum, it is also associated with the occurrence of infectious and metabolic diseases, as well as environmental stress. It is important to profitably manage acidosis and laminitis since both are a consequence of maximizing energy intake, milk production, and found in well managed herds. Critical areas of management include: feeding and management practices, attention to cow comfort, routine hoof trimming (twice/year), maintenance of adequate body condition, etc.
Additional Readings
WCDS-1995:
Metabolic Diseases - The Symptoms of a Greater Problem?
Applied Dairy Science Course - University of Alberta:
Metabolic and
Digestive Diseases of Dairy Cattle
References
1. Bargai, U., I. Schamia, A. Lublin and E. Bogin. 1992. Winter outbreaks of laminitis in dairy calves: etiology and laboratory, radiological and pathological findings. Vet. Rec. 131:411.
2. Bazeley, K. and P.J. Pinset. 1984. Preliminary observation on a series of outbreaks of acute laminitis in dairy cattle. Vet. Rec. 115:619.
3. Beauchemin, K.A., B.I. Farr, L.M. Rode and G.B. Schaalje. 1994. Effects of alfalfa silage chopped length and the supplementary long hay on chewing and milk production of dairy cows. J. Dairy Sci. 77:1526.
4. Bergsten C. 1994. Hemorrhages of the sole horn of dairy cows as a retrospective indicator of laminitis: an epidermiological study. Acta Vet. Scand. 35:55.
5. Boosman, R., J. Koeman and R. Knap. 1989. Histopathology of the bovine pododerma in relation to age and chronic laminitis. J. Vet. Med. 36:438.
6. Boosman, R., F. Nemeth, E. Gruys and A. Klarenbeek. 1989. Arteriographical and pathological changes in chronic laminitis in dairy cattle. Vet. Quart. 11:144.
7. Chavance, J. 1946. Histamine theory and treatment of laminitis. Vet. Med. 41:199.
8. Colan-Ainsworth, P., G.A., Lunn, R.C., Thomas and R.G. Eddy. 1989. Behavior of cows in cubicles and its possible relationship with laminitits in replacement heifers. Vet. Rec. 125:573.
9. Dewes, H. F. 1978. Some aspects of lameness in dairy herds. New Zealand Vet. J. 26:147.
10. Dirksen, G. 1989. Rumen function and disorders related to production disease. Pages 350-361 in proc. VII International Conference. Dis. In Farm Animals, Ithaca, NY.
11. Dunlap, R. H. and P. B. Hammond. 1965. D-lactic acidosis of ruminant. Ann. N.Y. Acad. Sci. 119:1109.
12. Eddy, R. G. and C. P. Scott. 1980. Some observations on the incidence of lameness in dairy cattle. in Somerset. Vet. Rec. 106:140.
13. Fulton, W., T.J. Klofenstein and R.A. Britton. 1979. Adaptation to high concentrate diets by beef cattle. I. Adapation to corn and wheat diets. J. Anim. Sci. 49:775
14. Garrett, E. F., M. N. Pereira, L. E. Armentano, K. V. Nordlund and G. R. Oetzel. 1995. Comparison of pH and VFA concentration of ruminal fluid from dairy cows collected through a rumen cannula vs ruminocentesis. J. Dairy Sci. Suppl. 1,78:299.
15. Greenough, P.R., F. J. MacCallum and A. D. Weaver. 1972. Lameness in cattle. L. B. Lippincott, Philadelphia and Toronto.
16. Greenough, P.R. and J.J. Vermunt. 1991. Evaluation of subclinical laminitis in a dairy herd and observations on associated nutritional and management factors. Vec. Rec. 128:11.
17. Huber, T. L. 1971. Effect of acute indigestion of compartmental water volumes and osmolarity in sheep. Am. J. Vet. Res. 32:887.
18. Kaufmann, W., H. Hagemeister and G. Dirksen. 1980. Adaptation to changes in dietary composition, level and frequency of feeding. Page 587 in Physiology and metabolism of ruminants. ed. Ruckebusch and Thivend. AVI Pousch and Co., Westport, CT.
19. Livesey, C.T. and F.L.. Fleming. 1984. Nutrtional influences on laminitis, sole ulcer and bruised sole in freisian cows. Vet. Rec. 114:510.
20. Logue, D.N., Lawson, A. and Roberts, D. 1989. The effect of two different protein sources in the diet upon the incidence and prevalence of lameness in dairy cattle.
21. Mackie, R. I. and F. M. C. Gilcrest and S. Heath. 1984. In vivo study of ruminal microorganisms influencing lactate turnover and its contribution to volatile fatty production. J. Agric. Sci. 103:37.
22. Maclean, C. W. 1971. The histopathology of laminitis in dairy cows. J. Comp. Pathol. 81:25.
23. Manson, F.J. and J.D. Leaver. 1988. The influence of concentrate amount on locomotion and clinical lameness in dairy cattle. Anim. Prod. 47:185.
24. Manson, F.J. and J.D. Leaver. 1988. The influence of dietary protein intake and of hoof trimming on lameness in dairy cattle. Anim. Prod. 47:191.
25. Manson, F.J. and J.D. Leaver. 1989. The effect of concentrates: silage ratio and hoof trimming on lameness in dairy cattle. Anim. Prod. 49:15.
26. Mortensen, K. 1994. Bovine laminitis (diffuse aseptic pododermitis) clinical and pathological findings. Page 210-226 in 8th Intern. Symp. on Disorders of the Ruminant Digit and Intern. Conf. on Bovine Lameness. Banff, Alberta, Canada.
27. Nagaraja, T. G. and G. Town. 1990. Ciliated protozoa in relation to ruminal acidosis and lactic acid metabolism. in Page 187-194. Rumen ecosystem: microbial metabolism and regulation, R. Oneora, H. Minato and H. Itabashi ed., Springer, Verlag, NY.
28. Nilsson, S.A. 1963. Clinical morphological and experimental studies of laminitis in cattle. Acta. Vet. Scand. 4 (Suppl. 1):1.
29. Nocek, J.E. 1987. Characterization of in situ dry matter and nitrogen in various corn grain forms. J. Dairy Sci. 70:2291.
30. Nocek, J.E. 1992. Feeding sequence and strategy effects on ruminal environment and production performance in first lactation cows. J. Dairy Sci. 75:3100.
31. Nocek, J.E. 1994. Hoof Care for dairy cattle. Hoard's Dairyman. Fort Atkinson, WI ISBN 0-932147-15-1.
32. Nocek, J.E. 1996. Bovine acidosis: implications in laminitis. J. Dairy Sci. (submitted).
33. Nocek, J. E., C. W. Heald, and C. E. Polan. 1984. Influence of ration of physical form and nitrogen availability on ruminal morphology of growing bull calves. J. Dairy Sci. 67:334.
34. Nocek, J.E. and J.B. Russell. 1988. Protein and energy as an integrated system. Relationship of ruminal protein and carbohydrates availability to microbial synthesis and milk production. J. Dairy Sci. 71:2070.
35. Nocek, J.E. and S. Tamminga 1991. Site of digestion of starch in the gastro-intestinal tract of dairy cows and its effect on milk yield and composition. J. Dairy Sci. 74:3598.
36. Norlund, K. V. 1995. Herd based rumenocentesis: a clinical approach to the diagnosis subacute rumen acidosis. Pages 1-11 in Proc. of the Northeast Medical Symposium. Ed. R. Saltman. Syracuse, NY.
37. Norlund, K. V. and E. F. Garrett. 1994. Rumenocentesis: a technique for collecting rumen fluid for the diagnosis of subacute rumen acidosis in dairy herds. The bovine practitioner 28:109
38. Ossent, P., C.J. Lischer. 1994. Theories on the pathogenesis of bovine laminitis. Pages 207-209 In: 8th Intern. Symp. on Disorder of the ruminant digit and Intern. Conf. on Bovine Lameness. ed. P.R. Greenough, Banff, Alberta, Canada.
39. Philipot, J.M., P. Pluvinage and F. Luquet. 1994. Clinical characterization of a syndrome by ecopathology methods: an example of dairy cow lameness. Vet. Res. 25:239.
40. Poore, M.H., J.A. Moore, R.S. Swingle, T.E. Eck & W.H. Brown. 1993. Response of lactating Holstein cows to diets varying in fiber source and rumen starch degradability. J. Dairy Sci. 76:2235.
41. Prentice, D. E. and P. A. Neal. 1972. Some observations on the incidence of lameness in dairy cattle in West Cheshire. Vet. Rec. 91:1.
42. Rowland, G.J., A.M. Russell and L.A. Williams. 1985. Effects of stage of lactation, month, age, origin and heart girth on lameness in dairy cattle. Vet. Rec. 117:576.
43. Russell, A. M., J. G. Rollands, R. S. Shaw and A. D. Weaver. 1982. Survey of lameness in British cattle. Vet. Rec. 111:155.
44. Russell, J. B. 1986. Ecology of rumen microorganisms: energy use. Page 74 in Aspects of digestive physiology and ruminates. Ed. A. Dobson and M. Dobson. 30th International Congress of the International Union of Physiological Sciences. Cornell University Press.
45. Russell, J. B. and T. Hino. 1985 . Regulation of lactate production in streptococcus bovis: A spiraling effect that contributes to rumen acidosis. J. Dairy Sci. 68:1712.
46. Sarwar, M., J.L. Firkins, and M.L. Eastridge. 1992. Effects of varying forage and concentrate carbohydrates on nutrient digestibilities and milk production by dairy cows. J. Dairy Sci. 75:1533.
47. Theurer, C.D. 1986. Grain processing effects on starch utilization by ruminants. J. Anim. Sci. 63:1649.
48. Tranter, W.P. and R.S. Morris. 1991. A case study of lameness in three dairy herds. N. Vet. J. 39:88.
49. Whitaker, D. A., J. M. Kelly and E. J. Smith. 1983. Incidence of lameness in dairy cows. Vet. Rec. 113:60.