Introduction
Ruminal microbes do not provide enough protein for maximum milk production. Dietary protein must escape ruminal degradation and pass to the small intestine to supply sufficient amounts of amino acids. A knowledge of amino acid requirements is important to minimize wastage of dietary protein and to optimize productivity.
Diet formulation systems described by NRC (15), ARC (1) and INRA (11) require partitioning dietary protein into fractions degraded in the rumen (DIPIP) and resistant to ruminal degradation (UIPIP). In vivo, in situ and in vitro methods are used for partitioning dietary protein into DIPIP and UIPIP.
Tabular values in NRC (15) are based on in vivo measurements with cattle fitted with cannulas in the abomasum or duodenum. Feed intake in most experiments was about two percent of body weight. High producing dairy cows consume feed at three to four percent of body weight. Because rate of passage of feed ingredients increases as feed intake increases, UIPIP of diets fed to lactating cows is probably greater than in NRC (15).
Alternatives to in vivo experiments are in situ fermentation of feeds and in vitro incubation with proteolytic enzymes. Both methods have problems. Bacteria can enter nylon bags suspended in the rumen and contaminate the undigested residue. Unless undigested residues are corrected for bacterial contamination, UIPIP will be over estimated. Like in vivo estimates, in situ methods are costly and are not readily adaptable for routine feed analyses. Enzymatic methods offer promise for routine feed analyses. However, saturation of enzyme upon substrate results in faster rates of protein degradation than occurs in the rumen. Reducing enzyme levels to limit rates of degradation can cause small variations in enzyme activity or quality which produce large analytical errors. In addition, optimal exposure time of different feed types to enzymes has not been established completely. Nevertheless, Krishnamoorthy et al. (12) obtained a good correlation (R2=0.84) between in vivo UIPIP and UIPIP estimated from in vitro incubation with Strep griseus protease.
In this report we use the Cornell Net Carbohydrate and Protein Model expanded to include amino acids (9,16,18,20) to estimate feed protein fractions, flows of protein and amino acids to the duodenum, absorption of protein and amino acids from the small intestine, and amino acid balance of example diets.
Partition of Feed Protein
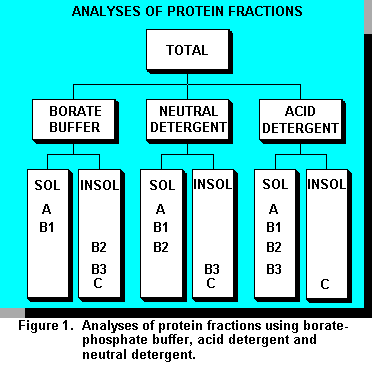 The detergent system developed by Goering and Van Soest (10) for analyses of carbohydrates in
conjunction with extraction with borate-phosphate buffer (9) offers a system to describe protein
fractions ( A, B1, B2, B3, and C, Figure 1) that, when altered in diets, will influence animal
responses (21).
The detergent system developed by Goering and Van Soest (10) for analyses of carbohydrates in
conjunction with extraction with borate-phosphate buffer (9) offers a system to describe protein
fractions ( A, B1, B2, B3, and C, Figure 1) that, when altered in diets, will influence animal
responses (21).Fractions A and B1 are soluble in borate-phosphate buffer. These can be partitioned further by extraction with TCA. Fractions B2, B3, and C are insoluble in borate-phosphate buffer. Extraction with neutral detergent prepared without sodium sulfite isolates fractions A, B1 and B2 (soluble in neutral detergent) from Fractions B3 and C (insoluble in neutral detergent). Acid detergent partitions proteins in fraction C (insoluble in acid detergent) and fractions A, B1, B2, and B3 (soluble in neutral detergent). Fraction B2 is calculated as the difference between borate-phosphate buffer insoluble protein and neutral detergent insoluble protein. Fraction B3 is the difference between neutral detergent insoluble protein and acid detergent insoluble protein. Proteins insoluble in acid detergent are fraction C.
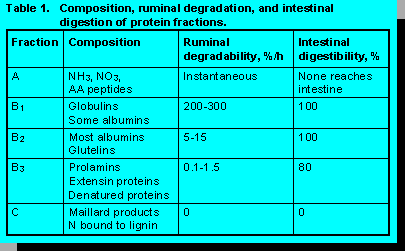
Composition, ruminal degradation, and intestinal digestion of protein fractions are in Table 1. Fraction A contains ammonia, nitrates, amino acids, and peptides; ruminal degradation is assumed to be instantaneous and none reaches the small intestine. B1 consists of globulins and some albumins. Because ruminal degradation is 200 to 300 %/hr, only small amounts reach the small intestine, but intestinal digestibility of B1 proteins is complete. B2 proteins contain most of the albumins and glutelins. Ruminal degradation is 5 to 15 %/hr with intestinal digestibility of 100%. B3 proteins are prolamins, extensin proteins (cell wall associated proteins), and heat denatured proteins that did not undergo the Maillard reaction. They are degraded in the rumen at 0.1 to 1.5 %/hr; intestinal digestibility is 80%. Fraction C proteins consist of Maillard reaction proteins (heat damaged protein) and nitrogen in with lignin. They are not degraded in the rumen and are considered indigestible in the intestine.
Partition of Feed Carbohydrate
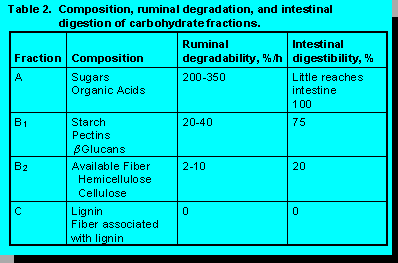 Composition, ruminal degradation, and intestinal digestion of carbohydrate fractions partitioned
by the detergent system (10) are in Table 2.
Composition, ruminal degradation, and intestinal digestion of carbohydrate fractions partitioned
by the detergent system (10) are in Table 2.Fractions A and B1 are soluble in neutral detergent. According to the model definitions, Fraction A consists of sugars whereas Fraction B1 consists of starch, pectins, and glucans (20).
Because ruminal degradation of Fraction A is 200 to 300 %/hr, less than 5% reach the intestine. Schofield and Pell (19) questioned these high rates of ruminal degradation. They reported degradation rates of 15 to 19 %/hr for neutral detergent solubles of forages. Neutral detergent solubles contain more than sugar. Also included are starch and pectic substances and non-carbohydrates such as protein, soluble phenolics, ash, and lipids. However, even when these lower rates are applied only to Fraction A, less than 20 to 25% will escape ruminal fermentation. The limited amount of Fraction A that escapes ruminal fermentation is completely digested in the intestine.
Ruminal degradation of Fraction B1 is 20 to 40%/hr. It varies depending upon the source of starch and methods of processing and storage. Rates of fermentation of starch in grains is wheat > barley > corn > sorghum. Fine grinding, steam flaking, and fermentation of high moisture grains increase rates of starch fermentation. Between 70 and 90% of feed starch is fermented in the rumen. Rumen escape starch has an intestinal digestibility of 75%.
Hemicellulose, cellulose, and lignin are insoluble in neutral detergent. The model partitions fiber into available (B2) and unavailable fractions on the basis of lignin and fiber. Unavailable fiber is lignin x 2.4. It represents the material remaining after 72 hr of in vitro fermentation (13). Lignin does not simply impede digestion by encrusting or covering nutrients. Instead, lignin protects associated carbohydrate from digestion. The mechanism is not entirely understood (22).
Ruminal degradation of Fraction B2 is 2 to 10%/hr. Between 30 and 70% of available fiber (Fraction B2) is fermented in the rumen. Rumen escape available NDF has a low intestinal digestibility (20%). Unavailable fiber (Fraction C) is not fermented in the rumen nor is it digested in the intestine.
The model predicts that 25 to 50% of feed fiber (Fraction B2 plus Fraction C) is fermented in the rumen. Because Fraction C (unavailable fiber) is defined as lignin x 2.4, the proportion of lignin in NDF has a greater impact on ruminal fermentation of feed fiber than the ruminal degradation rate of available fiber (Fraction B2).
Partition of feed carbohydrate is a weak link in The Net Carbohydrate and Protein System. This is especially true for Fraction A which is defined as sugar, but also contains silage organic acids. During silage fermentation, some of the soluble non-cell wall components are metabolized primarily to lactic and acetic acids. These organic acids are useful to the animal as a component of metabolizable energy, but are depleted fermentable sources of ATP for microbial growth (22). Thus, ensiling has little effect on feed energy values, but can affect protein nutrition substantially by decreasing bacterial protein production.
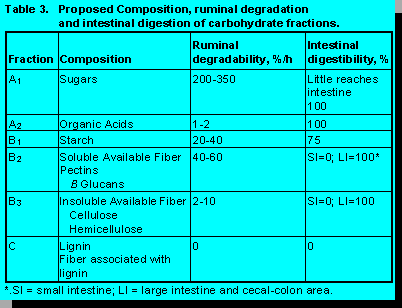
An alternative scheme for partition of feed carbohydrate is proposed in Table 3. Not only does it address the partition of silage organic acids from sugars, but it also allows representation of pectins and B glucans in a fraction designated soluble fiber.
Metabolizable Protein and Amino Acids
Metabolizable protein and amino acids are the sum of intestinally digested escape feed protein and bacterial protein. Protein fractions, intestinal protein, metabolizable protein, metabolizable methionine, lysine and isoleucine, metabolizable methionine(Met/MP) and lysine (Lys/MP) as percentages of metabolizable protein for 48 feed ingredients are in Tables 5 to 12.
Feed Protein
Protein fractions affect both ruminal escape and digestion of feed protein in the small intestine. Increasing Fractions B2, B3, and C will increase undegradability, but increasing Fraction C will decrease intestinal digestibility. Ensiling either forages (Tables 5 and 6) (Click here to view Table 5). (Click here to view Table 6). or grains (Table 7)(Click here to view Table 7). increases Fraction A at the expense of Fraction B2 so that ruminal escape is reduced. With forages, ensiling usually increases Fraction C so intestinal digestibility is reduced (Table 5). The effect of heat is demonstrated by comparing raw versus roasted soybeans (Table 10) (Click here to view Table 10). . Roasting soybeans reduces Fraction A and increases Fraction B3 at the expense of Fraction B1 so that undegradability is increased. Because Fraction C is not affected greatly, intestinal digestibility is not changed by roasting. On the other hand, brewers and distillers grains (Table 11) (Click here to view Table 11). are high in undegraded protein, but intestinal digestibility may be reduced because of the proportion of Fraction C. In general, intestinal digestibility of undegraded protein in protein meals, whole seeds, marine and animal proteins is high (Tables 10, 11 and 12) (Click here to view Table 12). . Ruminal undegradability of marine and animal proteins, however, is higher because they contain higher proportions of Fraction B3. Recently, Casamiglia and Stern (6) cautioned that intestinal digestibility of high bypass proteins can be variable.
Bacterial Protein
 Only carbohydrates or products of carbohydrate fermentation provide energy (ATP) at rates
sufficient for growth of most ruminal microbes (14). Thus, the amount of metabolizable protein
and amino acids derived from bacteria depends primarily on the amount and ruminal
fermentability of feed carbohydrate.
Only carbohydrates or products of carbohydrate fermentation provide energy (ATP) at rates
sufficient for growth of most ruminal microbes (14). Thus, the amount of metabolizable protein
and amino acids derived from bacteria depends primarily on the amount and ruminal
fermentability of feed carbohydrate.
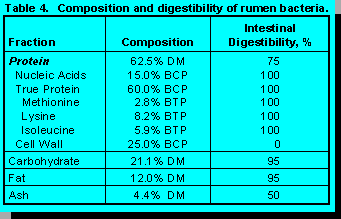
Composition and digestibility of rumen bacteria are in Table 4. Bacteria contain 62.5% crude protein, but 25% of the protein is in cell walls and is indigestible in the small intestine. This fraction is like Fraction C in feed protein. Nucleic acids (15% BCP) and true protein (60% BCP) are completely digested in the intestine, but only bacterial true protein is a source of amino acids.
Metabolizable Amino Acids
Metabolizable methionine and lysine are expressed as quantities in feed ingredients and as percentages of metabolizable protein. The former are needed to formulate rations using the classical factorial method (16) while the latter are needed to formulate rations on an ideal protein method (17). Minimum levels appear to be 2.15 to 2.20% methionine and 6.6 to 6.8% lysine. Ruminal bacterial true protein contains 2.8% methionine and 8.2% lysine (Table 4).
Feed Ingredients
Forages. In high producing cows, forages are 40 to 50% of dry matter intakes. They are important sources of highly digested fiber needed to maintain rumen function. As discussed below, their delivery of metabolizable protein and amino acids can be quite different than previously thought.
Alfalfa. Protein fractions and amino acids supplied by three qualities of alfalfa stored as hay and as silage are in Table 5. (Click here to view Table 5). Ruminal escape of alfalfa protein is mainly determined by the amount of soluble protein (Fractions A and B1). More alfalfa protein escapes ruminal fermentation when fed in the form of hay than as silage, but with both forms escape decreases as concentration of crude protein increases. Intestinal digestibility of escape protein is higher in hay than in silages because silage fermentation increases Fraction C.
Bacterial protein production is inversely related to concentration of NDF reflecting higher concentrations of carbohydrate Fractions A and B1. To allow for the metabolism of sugar to organic acids during silage fermentation, we decreased the degradation rate of Fraction A to 4 %/hr. This decreased ruminal fermentation from 98% for alfalfa hay to 40 to 45% for alfalfa silage. With this adjustment, 55 to 60% of Fraction A in silages was placed into organic acids that, while contributing to metabolizable energy, did not contribute to bacterial growth. Consequently, bacterial protein from alfalfa silage was only about 50% of the bacterial protein produced when alfalfa hay was fed. Van Soest (22) calculated that ATP yield of ensiled alfalfa was 46% of the ATP yield of fresh alfalfa while metabolizable energy yield was 86%.
Because ensiling increases soluble protein (Fractions A and B1) and unavailable protein (Fraction C) and results in decreased bacterial growth, metabolizable protein and amino acids arising from feeding alfalfa hay are about twice that observed when alfalfa is fed in the form of silage. Feeding systems other than INRA (11) assume that soluble matter in fermented feeds is the same as soluble matter in unfermented feeds. Thus, lower performance of animals fed rations containing high amounts of silage may be due to metabolizable protein and amino acid deficiencies.
Regardless of whether alfalfa is fed as hay or silage, alfalfa provides only small amounts of metabolizable protein and amino acids. Increasing concentration of crude protein in alfalfa has little effect on delivery of metabolizable protein and amino acids. Alfalfa with 20% crude protein and 40% NDF is ideal for formulating rations for high producing dairy cows.
Other Silages. The silages in Table 6 (Click here to view Table 6). have high proportions of protein in Fraction A. This leads to low rumen escape of feed protein. Unavailable protein (Fraction C) in corn and sorghum silages is only 5 to 8% so intestinal digestibility of escape feed protein is 60 to 70%.
To compensate for formation of organic acids during silage fermentation of corn, sorghum, and barley, ruminal degradation of carbohydrate Fraction A was reduced to 3 %/hr. This reduced ruminal fermentation to 40 to 50%, allowing 50 to 60% of Fraction A to be in the form of organic acids. Because of high concentrations of carbohydrate Fractions A, and B1 in conjunction with low amounts of unavailable fiber (Fraction C), growth of rumen bacteria was high.
Because of high bacterial growth, metabolizable protein supplied by corn, sorghum, and barley silages is 65 to 90% of feed protein. Delivery of methionine, lysine, and isoleucine is greater than from alfalfa. Methionine and lysine as percentages of metabolizable protein are above minimum levels.
Grains. Grains are usually categorized as energy-yielding ingredients. However, because of their types and fermentability of carbohydrates, grains provide substantial amounts of metabolizable protein and amino acids through growth of bacteria. Table 7 (Click here to view Table 7). contains information on corn. Information on other grains is in Table 8. (Click here to view Table 8). Because protein Fractions A and B1 are low, ruminal escape of feed protein is high. Heat from steam flaking can cause further increases in rumen escape, but fermentation of high moisture grains increases Fraction A and decreases ruminal escape. Low Fraction C results in high digestibility of intestinal feed protein.
Bacterial growth is determined by the inherent rates of fermentation of starch in grains, but this can be adjusted by processing. Thus, grinding, steam flaking, and fermentation of high moisture grains increase starch fermentability and increase growth of rumen bacteria. Metabolizable protein supplied by corn, barley, wheat, and steam flaked sorghum is greater than the concentration of protein in the diet primarily due to greater bacterial protein production.
Grain proteins are low in lysine. Thus although grains promote substantial bacterial growth, Lys/MP is below critical levels. Exceptions are high moisture corn and ground wheat. With high moisture corn, rumen escape is decreased and bacterial protein is a greater proportion of metabolizable protein. Starch in ground wheat is highly fermentable so that bacterial growth is high. Corn, barley, and wheat have Met/MP that are above minimum levels.
Carbohydrate Byproducts. Byproducts in Table 9 (Click here to view Table 9). contain 80 to 90% carbohydrate. Composition of carbohydrates is variable. For example, almond hulls, beet pulp, and citrus pulp are high in non-fiber carbohydrate (Fractions A and B1), whereas soybean hulls are high in available fiber (Fraction B2). These byproducts are used as economical energy-yielding ingredients and as extenders of forage fiber.
Because these ingredients supply energy for bacterial growth, they can contribute to supplies of metabolizable protein and amino acids.
Oil Seeds. Protein fractions and supplies of metabolizable protein and amino acids of commonly fed oilseeds and their meals are in Table 10. (Click here to view Table 10).
Mainly because of low amounts of Fraction B3, rumen escape of protein in soybean meal, whole cottonseed and sunflower meal is only 30 to 35%. Cottonseed meal and canola meal have similar protein fractions, but because ruminal degradation of Fraction B2 is lower, ruminal escape is higher. Ruminal escape of protein in roasted soybeans is higher than protein in soybean meal because heat increases Fraction B3. Ruminal escape of protein in oilseed meals varies substantially as a function of feed intake because the high amount of Fraction B2 has rates of ruminal degradation (5 to 11 %/hr) that are similar to rates of feed particle passage (5 to 8 %/hr). Low concentrations of Fraction C result in intestinal digestibilities of 80 to 90%.
Carbohydrate concentrations of 30 to 50% do not promote large amounts of bacterial growth. Thus, metabolizable protein and amino acids provided by oilseeds come mainly from feed protein.
Oilseeds are low in methionine and Met/MP is below critical levels. Supplies of lysine are modest, but with the exception of canola meal Lys/MP is below minimum levels.
Protein Byproducts. Information on brewers grains, distiller grains, corn gluten meal, and corn gluten feed is in Table 11.Click here to view Table 11.
With the exception of corn gluten meal and corn gluten feed, the high concentration of Fraction B3 is responsible for ruminal escape of about 60% of feed protein. The high ruminal escape (60%) of protein in corn gluten meal is due to the high concentration (85%) of Fraction B2. Corn gluten feed contains a high amount (45%) of Fraction A so ruminal escape is low (31%). Appreciable amounts of unavailable protein (Fraction C) can decrease intestinal digestibility.
Because these protein byproducts contain low concentrations of fermentable carbohydrate (Fractions A and B1), bacterial growth is low. Consequently, 70 to 90% of metabolizable protein and amino acids are derived from feed proteins.
These protein byproducts are low in Methionine and lysine and thus have very low Met/MP and Lys/MP. Corn gluten meal is a good source of methionine and isoleucine, but the low Lys/MP makes it difficult to use when formulating amino acids on the ideal protein method.
Animal and Marine Proteins. As shown in Table 12, Click here to view Table 12. animal and marine proteins are low in Protein Fractions A, B1, and C and high in Protein Fractions B2 and B3.This results in high ruminal escape (60 to 80%) of feed protein and high (80 to 90%) intestinal digestibility of escape protein. However, as noted earlier, Casamiglia and Stern (6) cautioned that intestinal digestibility of byproduct and rendered proteins can be low.
Absence of rumen fermentable carbohydrate in animal and marine proteins precludes bacterial growth so that metabolizable protein and amino acids are derived only from feed protein.
Blood meal provides the greatest amount of lysine and the highest Lys/MP. Quantity of methionine is modest, but Met/MP is low. Based on supplies of methionine and lysine and Met/MP and Lys/MP, fish meal is the best protein ingredient. Feather meal is high in isoleucine, but Met/MP and Lys/MP are low. Low Met/MP and Lys/MP in meat and bone meal and meat meal make it difficult to use the ingredients in rations formulated on the basis of ideal protein.
Ration Formulation
Example Rations
In Table 13 (Click here to view Table 13). are example rations for high producing cows that were formulated using the Net Carbohydrate and Protein Model modified to contain an optimizer (5). By using high cost dummy variables, the autobalancer always gives a solution, even if nutrient constraints cannot be met.
Corn silage and alfalfa haylage were constrained to provide 45% forage in the ration with a minimum of 2.5 kg alfalfa haylage. Soybean hulls were an additional source of highly fermented fiber. Grain sources were ground corn and barley (maximum 3 kg). Protein sources were soybean meal, blood meal, fish meal, and corn gluten meal. Whole cottonseed was available as an additional source of energy, fiber, and protein. Megalac Plus was available as a source of methionine and energy. So that Megalac Plus would be selected as a source of methionine and not for energy, Megalac was also available.
Nutrients constrained included metabolizable energy (100% requirement), metabolizable protein (100% requirement), metabolizable isoleucine (95 to 110% requirement), ruminal peptides and ammonia (100 to 150% requirement), NDF (30 to 34% ration dry matter), NFC (40% ration dry matter), and fat (0 to 6% ration dry matter).
Requirements for absorbed essential amino acids were defined using the classical factorial method (16) and for absorbed methionine and lysine, an ideal protein method (17) was also used. The factorial method requires knowledge of the amino acid content of products and the efficiency of amino acid use. Amino acid content of milk and tissues can be estimated reliably, but estimates of the efficiency of amino acid use is difficult and variable. The ideal protein method of Rulquin and Verite (17) is based on responses of milk protein to methionine and lysine expressed as percentages of metabolizable amino acids. Because milk protein yield appears to be dramatically reduced when rations provide less than 2.15 to 2.20% methionine or 6.6 to 6.8% lysine, these levels were used as minimums.
Six rations were formulated, using a 3 x 2 factorial design. Methionine and lysine requirements were calculated using the classical factorial approach and the ideal protein method at two concentrations of methionine and lysine. The three estimates of methionine and lysine requirements were met with rations where forage Fraction A degradation rate were reduced to accommodate metabolism of sugar to organic acids during silage fermentation and where no reductions of Fraction A degradation rate were imposed.
Formulations using the factorial approach produces rations that are below minimum for Met/MP and Lys/MP. At Met/MP=2.15 and Lys/MP=6.60, supplies of methionine and lysine are 6% higher than predicted by the factorial method. These excesses increase to 9 to 14% when ideal protein constraints are Met/MP=2.20 and Lys/MP=6.80.
When Carbohydrate Fraction A degradation rate was reduced, rations contained more crude protein with greater bypass. This reflects lower bacterial growth because of reduced supplies of fermentable carbohydrate.
Selection of feed ingredients by the optimizer was interesting. Barley was selected when forage sugar degradation rates were reduced. This was an attempt to compensate for decreased rumen fermented carbohydrate and reflects the higher ruminal fermentation of starch in barley versus corn. Corn gluten meal was selected with the factorial amino acid method, but not when methionine and lysine were met by the ideal protein method. This reflects the good supply of isoleucine, but the poor Lys/MP of corn gluten meal. Megalac Plus was selected when rations were formulated on the basis of the ideal protein method while Megalac was selected when the factorial method was used. This reflects the need for methionine to satisfy Met/MP.
Sources of Metabolizable Protein and Amino Acids
Metabolizable protein, methionine, lysine, and isoleucine provided by ingredients in ration 3 from Table 13 are in Table 14. Corn silage, corn, and barley are 58% (Table 14) (Click here to view Table 14). of DMI and provide about 50% of the metabolizable protein and amino acids. These ingredients are low in crude protein, so most of their metabolizable protein (68%) and their amino acids (80 to 90%) are derived from bacteria. Alfalfa haylage is 11% of DMI, but only provides 5 to 7% of metabolizable protein and amino acids with more coming from bacteria than from feed. Soybean meal, blood meal, and fish meal are 11% of DMI, but provide 34% of the metabolizable protein, 25% of the methionine, 37% of the lysine, and 28% of the isoleucine. Because there is little bacterial growth on these ingredients, most of the metabolizable protein and amino acids come from feed.
Rumen Protected Amino Acids
The primary methods developed to prevent fermentative digestion of amino acids are structural manipulation to produce amino-acid analogs and coating with resistant materials.
The main amino acid analogs evaluated are methionine hydroxy analog, N-(hydroxymethyl) DL-methionine calcium, and mono plus di-N-(hydroxymethyl)- L-lysine calcium. Methionine hydroxy analog appears to be more resistant to fermentative digestion than methionine, but substantial amounts do not appear to bypass the rumen (7).
Amino acids have been coated with polymeric compounds, formalized protein, fat, mixtures of fat and calcium, mixtures of fat and protein, and with calcium salts of long chain fatty acids (7,8).
 Recently, a new product (Megalac PlusR ) was introduced (Church and Dwight Co., Inc.
Princeton NJ). Megalac PlusR contains 3% methionine hydroxy analog added during the
manufacture of MegalacR. The calcium salts of long chain fatty acids gives additional protection
to methionine hydroxy analog. In a study on a commercial dairy (8), cows fed 0.5 kg/d Megalac
PlusR versus MegalacR produced an additional 1.7 kg/d milk with modest increases in
concentration of milk protein and fat (Table 15). In primiparous cows, milk yield decreased (3.8
kg/d), but there was a substantial increase in concentration of milk protein (0.12 percentage units)
and a modest increase in milk fat (0.08 percentage units).
Recently, a new product (Megalac PlusR ) was introduced (Church and Dwight Co., Inc.
Princeton NJ). Megalac PlusR contains 3% methionine hydroxy analog added during the
manufacture of MegalacR. The calcium salts of long chain fatty acids gives additional protection
to methionine hydroxy analog. In a study on a commercial dairy (8), cows fed 0.5 kg/d Megalac
PlusR versus MegalacR produced an additional 1.7 kg/d milk with modest increases in
concentration of milk protein and fat (Table 15). In primiparous cows, milk yield decreased (3.8
kg/d), but there was a substantial increase in concentration of milk protein (0.12 percentage units)
and a modest increase in milk fat (0.08 percentage units).
Application
NRC (15), ARC (1), and INRA (11) presented frame-works based on the biology of nitrogen metabolism in ruminants that form the basis for calculating protein requirements of lactating dairy cattle. These systems detailed protein nutrition in terms of pools and transfer coefficients between pools. Because information needed for operation of detailed models of protein nutrition was not available, initial recommendations of protein requirements were estimated from static-aggregated models that only considered overall ruminal degradation of dietary protein and synthesis of microbial protein driven only by ruminally available energy. Although simplistic, these static-aggregated models were useful in educating dairy producers and their advisors on the dynamics of protein nutrition so that diets fed to high producing dairy cows usually now contain by-product feed ingredients that are resistant to ruminal degradation.
The Cornell Net Carbohydrate and Protein System for evaluating cattle diets (9,16,18,20) is a mix of empirical and mechanistic approaches that describe feed intake, ruminal fermentation of protein and carbohydrate, intestinal digestion and absorption, excretion, heat production, and utilization of nutrients for maintenance, growth, lactation, and pregnancy. The system can be applied at the farm level because diets are characterized according to fractions that are measured easily in most feed analyses laboratories. We have found the system to be especially valuable in estimating ruminal degradability of dietary protein and in determining whether ruminal microbes are provided with proper types and amounts of nitrogenous nutrients (i.e., ammonia, peptides). The system has also been useful in providing information on amino acid requirements and in identifying limiting amino acids.
Ultimately, nutrient requirements of dairy cattle will be based on quantitative and dynamic mathematical descriptions of biochemical reactions. The efforts of Baldwin et al. (2,3,4) demonstrate that biochemical data generated from tissue level experiments in vitro can be used to develop mechanistic whole-animal models that are useful in describing utilization of nutrients and nutrient requirements.
Additional Readings
Applied Dairy Science Course - University of Alberta:
Ruminal Digestion of carbohydrates, protein, and lipids.
References
1. ARC. 1980. The Nutrient Requirements of Ruminant Livestock. Commonwealth Agricultural Research Council. Slough, U.K.
2. Baldwin, R.L., J. France and M. Gill. 1987a. J. Dairy Res. 54:77.
3. Baldwin, R.L., J.H.M. Thornley and D.E. Beever. 1987b. J. Dairy Res. 54:107.
4. Baldwin, R.L., J. France, D.E. Beever, M. Gill and J.H.M. Thornley. 1987c. J. Dairy Res. 54:133.
5. Boston R. and W. Chalupa. 1994. Unpublished research. Univ. Pennsylvania. Kennett Square.
6. Casamiglia, S. and M.D. Stern. 1995. J. Anim. Sci. 73:1459.
7. Chalupa, William and Charles J. Sniffen. 1991. The Veterinary Clinics of North America-Food Animal Practice: Dairy Nutrition Management. Page 353. W.B. Saunders CO., Philadelphia.
8. Ferguson, J.D., W. Chalupa, N. Thomsen, D.T. Galligan and K. Cummings. 1993. J. Dairy Sci. 76(Suppl. 1):184.
9. Fox, D.G., C.J. Sniffen, J.D. O'Conner, J.B. Russell; and P.J. Van Soest. 1992. J. Anim. Sci 70:3578.
10. Goering, H.K. and P.J. Van Soest. 1970. Agric Handbook No. 379. ARS, USDA, Washington DC.
11. INRA. 1989. Ruminant Nutrition. Recommended allowances and feed tables. (Ed. J. Jarrige). John Libbey Eurotext, London.
12. Krishnamoorthy, U., C.J. Sniffen, M.D. Stern and P.J Van Soest. 1983. Br. J. Nutr. 50:555.
13. Mertens, D.R. 1973. Application of Theoretical Mathematical Models to Cell Wall Digestion and Forage Intake in Ruminants. Ph.D. Dissertation. Cornell Univ., Ithaca, NY.
14. Nocek, J.E. and J.B. Russell. 1988. J. Dairy Sci. 71:2070.
15. NRC. 1989. Nutrient Requirements of Dairy Cattle. Nat Res Council, Wash., D.C.
16. O'Connor, J.D., C.J. Sniffen, D.G.Fox and W. Chalupa. 1993.J. Anim. Sci.71:1298.
17. Rulquin, H. and R. Verite. 1993. Recent Advances in Animal Nutrition. Univ Nottingham Press.
18. Russell, J.B., J.D. O'Connor, D.G. Fox, P.J. Van Soest and C.J. Sniffen. 1992. J. Anim. Sci.70:3551.
19. Schofield, P. and A.N. Pell. 1995. J. Anim. Sci. 73:3455.
20. Sniffen, C.J., J.D. O'Connor, P.J. Van Soest, D.G. Fox and J.B. Russell. 1992. J. Anim. Sci. 70:3562.
21. Sniffen, C.J., M.B. Roe, A.P. Rafferty, J.D. O'Connor, D. Fox and W. Chalupa. 1990. Proc. Univ. Guelph Nutr. Conf.
22. Van Soest, P.J. 1994. Nutritional Ecology of the Ruminant. Cornell University Press, Ithaca.