Somatotropin to Extend Lactation
At the present time low cull cow prices and high replacement heifer costs have created a significant cash flow squeeze on dairy operations. Presently, it takes four cull cows to generate sufficient income to buy one replacement. At typical culling rates of 30%, it is easy to understand why many dairy farmers are having trouble maintaining their herd size. Keeping cows in the dairy herd can be very profitable if their production can be maintained. Therefore, using somatotropin to extend lactation well beyond 305 days might be a viable commercial opportunity. However, to be significant, this strategy would also need to utilize delayed breeding to avoid negative effects of pregnancy on the lactation performance. Delayed breeding with somatotropin use might then allow the producer to extend the lactation to 610 days rather than the standard 305. Data on lactation performance of somatotropin-treated open cows is now being collected at Cornell University by Dr. Dave Galton to examine this hypothesis. He is evaluating somatotropin use to extend lactation in open cows in several New York dairies and plans to summarize their performance at the American Dairy Science Meetings in 1996.
Somatotropin Use in Mastitis
Although it has been demonstrated that there is a slight increase in mastitis in lactating cows treated with somatotropin (45), this increase is associated primarily with the increase in milk yield. Somatotropin has been shown to have positive effects on the immune system (14). Burvenich et al. (10,11) and Vandeputte-Van Messom and Burvenich (37) demonstrated that somatotropin treatment shortened the mammary gland's recovery time following experimentally induced E. coli infection. Recovery was measured primarily as the rate and extent of restoration of the blood-milk barrier in inflamed quarters. Somatotropin treatment reduced milk sodium and chloride concentrations toward normal levels. Thus, cellular polarity and junctional complexes between lactating mammary cells were restored sooner in glands of cows treated with somatotropin. Other studies have demonstrated improved lymphoblastogenesis, immunoglobulin production, and increased neutrophil killing activity in somatotropin-treated cattle which indicates that somatotropin may improve the immune system response to a bacterial challenge (7,8,9,14). Additional work is needed to determine if there are therapeutic uses for somatotropin in treatment and recovery from mastitis.
Somatotropin for Prevention and Treatment of Metabolic Disease
Peripartum disorders involving periparturient hypocalcemia, reproductive disorders, and ketosis generally occur as a complex. Dystocia, milk fever, mastitis, retained placenta, metritis, LDA, and ketosis have been shown to be inter-related in peripartum dairy cows (15,16). For example, Curtis et al. (16) found that cows which had milk fever were 7.2, 4.0, 5.4, and 23.6 times more likely to suffer dystocia, retained placenta, clinical mastitis, or complicated ketosis (ketosis with an occurrence of at least one other clinical disease present concurrently or previously). Similarly, retained placenta, left displaced abomasum, and milk fever directly increased the risk of complicated ketosis by 16.4, 53.5, and 23.6 times (16).Ketosis itself is a metabolic disease affecting early lactation dairy cows. Symptoms include decreased milk production, nervousness or lethargy, and inappetance. Metabolic implications include decreased glucose concentration in blood, increased ketones in urine, and increased blood betahydroxybutyrate (25). Recent research suggests that increased fatty liver and decreased glycogen stores may have an effect on whether a cow suffers from clinical ketosis (30,38).
Peripartal treatment with bovine somatotropin has been demonstrated to increase bone calcium mobilization in peripartal dairy cows (19). Although milk fever was not improved in that study, somatotropin's effect on calcium metabolism could have ameliorated the related disorders of displaced abomasum and ketosis. Also of interest is the increase in gluconeogenesis, which is attributed to the direct effect on bST on the liver (5), whereas the increase in milk production is due to stimulation by IGF-1 at the mammary gland. Also, Vicini et al. (42) showed that while lactating dairy cows have relatively high bST levels in early lactation, IGF-1 concentrations are low.
Considerable research will be required to determine if somatotropin can be used as a prophylactic agent in prevention of metabolic disease or whether it may be a successful treatment for certain metabolic diseases such as ketosis and milk fever. However, there is sufficient positive data on somatotropin's mechanism of action to pursue these as real possibilities.
Somatotropin to Accelerate Growth and Age at First Calving
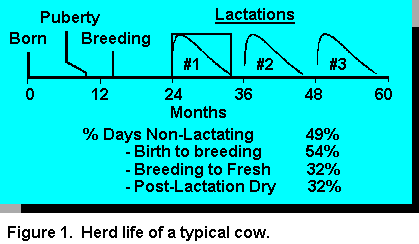 Traditionally, management of replacement heifers has received little attention in comparison to
overall dairy farm operations. However, the value of bringing vigorous heifers into production as
soon as is economically feasible has received greater attention. The value in this approach is
apparent when one considers the large portion of the typical dairy cow's herd-life that is prior to
her first lactation (Figure 1) (40).
Traditionally, management of replacement heifers has received little attention in comparison to
overall dairy farm operations. However, the value of bringing vigorous heifers into production as
soon as is economically feasible has received greater attention. The value in this approach is
apparent when one considers the large portion of the typical dairy cow's herd-life that is prior to
her first lactation (Figure 1) (40).Feeding high energy diets, by incorporating more concentrates, has improved efficiency of animal performance in most areas of animal agriculture. However, this approach has not been feasible in raising replacement heifers because of reduced milk yields in their first lactation. Lowered blood somatotropin and reduced parenchymal tissue, in mammary glands of animals fed high energy diets prior to puberty, have been observed. These changes may be involved in the etiology of the lowered production of heifers fed high energy dense diets (40).
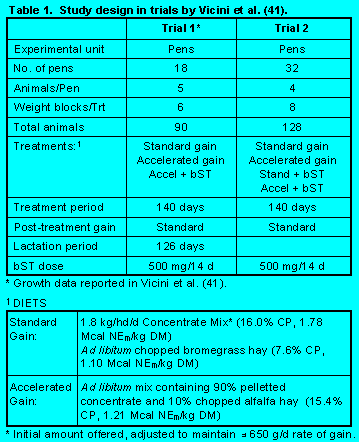
In a trial previously reported, Vicini et al. (41) (Table 1) administered somatotropin to heifers fed for accelerated growth prior to puberty. Weight gain was increased and days to puberty were reduced by the combination of high energy diets and somatotropin administration. Subsequent milk production of these heifers is shown in Figure 3. In a second trial, Vicini et al. (41) tested to see whether somatotropin would affect weight gain of heifers fed restricted quantities of feed.
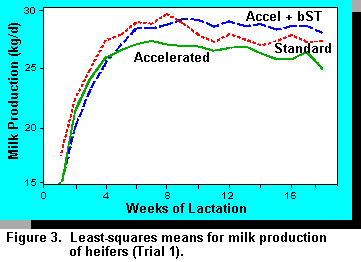
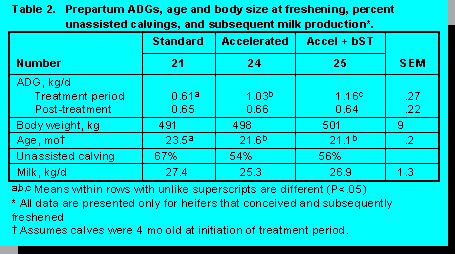
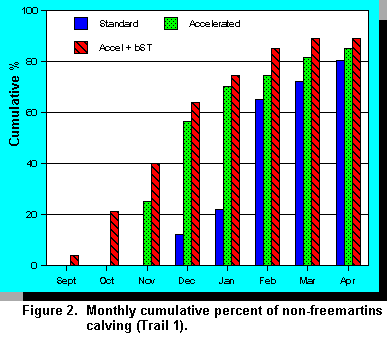
Trial 1. Throughout the treatment period mean rates of gain were higher when heifers were fed the accelerated diets and rates of gain were even higher when bST was administered (Table 2). Heifers reached puberty at a target body weight regardless of weight gain. Breeding was done on a pen basis when the average weight was 350 kg. Weight differences were maintained throughout the breeding period and gestation. Accelerated pre-pubertal rates of gain resulted in animals coming into production earlier (Figure 2) with no loss in milk production (Figure 3). Dystocia and other calving difficulties were not affected by treatments (P>.10).
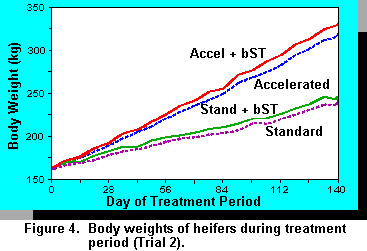
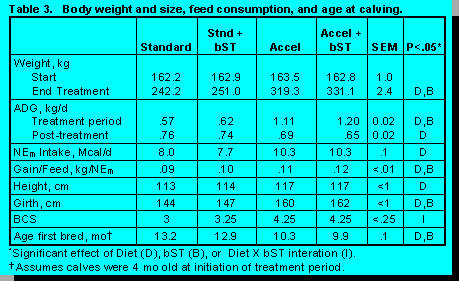
Trial 2. Heifer calves were tested in a similar design with the exception that an additional group was added to determine if bST treatment would increase growth of calves when fed restricted amounts of concentrates at lower rates of body weight gain. The high energy diets increased all measurements of body weight gain (Figure 4, Table 3) and similarly, bST increased body weight, ADG, and girth. Age at breeding was decreased by diet and bST.
To summarize:
Conclusions
A wide variety of therapeutic and prophylactic uses of somatotropin for dairy cattle are being tested. Final commercial value of these opportunities will be determined by the dairy industry and cost of regulatory approvals relative to their value. However, it is clear that opportunities exist to expand use of somatotropin beyond increasing milk yield. In some countries the potential for therapeutic use may be of more value to the dairy industry than use for increasing milk yield.
Additional Readings
Alberta Dairy Management Fact Sheet:Growth Hormone - 1. Introduction and Production Response
References
1. Adriaens, F.A., D.L. Hard, M.A. Miller, R.H. Phipps, R.H. Sorbet, R.L. Hintz and R.J. Collier. 1994. Domestic Animal Endo. 12:301-316.2. Adriaens, F.A., M.A. Miller, D.L. Hard, R.F. Weller, M.D. Hale and R.J. Collier. 1992. J. Dairy Sci. 75:472-480.
3. Bauman, D.E. and M.A. McGuire. 1994. Paradox of BST: Why Cows Don't Burnout. In: Proceedings Minnesota Dairy Health Conference. U. of Minn. St. Paul, MN pp. 27-40.
4. Bauman, D.E. and R.G. Vernon. 1993. Ann. Rev. Nutr. 13:437-461.
5. Bauman, D.E., C.J. Peel, W.D. Steinhour, P.J. Reynolds, H.F. Tyrrell, A.C.G. Brouwn and G.L. Haaland. 1988. J. Nutr. 118:1031-1040.
6. Britt, Jack. Personal communication.
7. Burton, J.L., B.W. McBride, B.W. Kennedy, J.H. Burton, T.H. Elsasser and B. Woodward. 1992. J. Dairy Sci. 74:1589-1598.
8. Burton, J.L., B.W. McBride, B.W. Kennedy, J.H. Burton, T.H. Elsasser and B. Woodward. 1992. J. Dairy Sci. 75:747-755.
9. Burton, J.L., B.W. McBride, B.W. Kennedy, J.H. Burton, T.H. Elsasser and B. Woodward. 1991. J. Dairy Sci. 74:916-928.
10. Burvenich, C., G. Vandeputte-Van Messom, E. Roets, J. Fabry and A.M. Massart-Leen. 1989. Effect of bovine somatotropin on milk yield and milk composition in periparturient cows experimentally infected with Escherichia coli. In: Use of Somatotropin in Livestock Production, Eds. K. Sejrsen, M. Vestergaard and A. Neimann-Sorensen. Elsevier Appl. Sci., New York, NY, pp. 277-284.
11. Burvenich, C., G. Vandeputte-Van Messom, E. Roets, A. Massart-Leen, R.M. Akers, R. Reynart-Van Sichem and L. Devrieze. 1988. Ann. Med. Vet. 132:601-606.
12. Chalupa, W. and D.T. Galligan. 1989. J. Dairy Sci. 72:2510-2524.
13. Cole, W.J., K.S. Madsen, R.L. Hintz and R.J. Collier. 1991. Theriogenology 36:573-595.
14. Comens-Keller, P.G., P.J. Eppard and R.J. Collier. 1995. Evaluation of somatotropin as a homeorhetic regulator of immunity. In: Animal Science Research and Development: Moving Toward a New Century, Ed. M. Ivan. Centre for Food and Animal Research, Agriculture and Agri-Food Canada, Ottawa, ON K1A 0C6, Canada, pp. 79-94.
15. Correa, M.T., C.R. Curtis, H.N. Erb, J.M. Scarlett and R.D. Smith. 1990. J. Dairy Sci. 73:1515-1524.
16. Curtis, C.R., H.N. Erb, C.J. Sniffen, R.D. Smith and D.S. Kronfeld. 1985. J. Dairy Sci. 68:2347-2360.
17. Effect of somatotropin on nutrient requirements of dairy cattle. 1994. In: Metabolic Modifiers: Effects on the Nutrient Requirements of Food Producing Animals. National Academy Press, Washington, D.C. pp. 23-29.
18. Elsasser, T.H. and N.C. Steele. 1992. Growth hormone directed nutrient partitioning: Immune system-pituitary gland communication. In: Pennington Cent. Nutr. Ser. 2, Science of Food Regulation. pp. 164-186.
19. Eppard, P.J., J.J. Veenhuizen, W.J. Cole, P.G. Comens-Keller, G.F. Hartnell, R.L. Hintz, P.K. Olsson, R.H. Sorbet, T.C. White, C.A. Baile and R.J. Collier. 1995. Effect of bovine somatotropin administered to periparturient dairy cowson incidence of metabolic disease. Submitted J. Dairy Sci.
20. Exon, J.H., J.L. Bussiere and J.R. Williams. 1990. Brain Behav. Immun. 4:118-128.
21. Gelato, M.C. 1993. Trends Endocrinol. Metab. 4:106-110.
22. Herrler, A., R. Einspanier, D. Schams and H. Niemann. 1994. Theriogenology 41:601-611.
23. Hoogendoon, C.J., S.N. McCutcheon, G.A. Lynch, B.W. Wickham and A.K.H. MacGibbon. 1990. Anim. Prod. 51:431-439.
24.Kuehner, L.F., D. Rieger, J.S. Walton, X. Zhao and W.H. Johnson. 1993. Theriogenology 40:1003-1013.
25. Littledike, E.T., J.W. Young and D.C. Beitz. 1981. J. Dairy Sci. 64:1465-1482.
26. Lucy, M.C., T.L. Curran, R.J. Collier and W.J. Cole. 1994.Theriogenology 41:561-572.
27. Lucy, M.C., R.J. Collier, M.L. Kitchell, J.J. Dibner, S.D. Hauser and G.G. Krivi. 1993. Biol. Reprod. 48:1219-1227.
28. Lucy, M.C., W.W. Thatcher, J.D. Savio, G. Danet-Desnoyers, M.T. Moser, L. Badinga, F.A. Simmen and R.J. Collier. 1992. J. Anim. Sci. 70:(Suppl.1):271.
29. Marsh, J.A., W.C. Gause, S. Sandhy and C.G. Scanes. 1984. Proc. Soc. Exp. Biol. Med. 175:351-360.
30.Mills, S.E., D.C. Beitz and J.W. Young. 1986. J. Dairy Sci. 69:352-361.
31. Minton, J. E. 1994. J. Anim. Sci. 72:1891-1898.
32.Rieger, D., J.S. Walton, M.L. Goodwin and W.H. Johnson. 1991. Theriogenology 35:863-868.
33. Snow, C.E. 1985. J. Immunol. (Suppl.2):776S-778S.
34. Stanisiewski, E.P. 1993. Somatotropin for increasing reproductivity performance in cattle. Patent Application. WO 9303756 A1.
35. Stanisiewski, E.P., L.F. Krabill and J.W. Lauderdale. 1992. J. Dairy Sci. 75:2149-2164.
36. Takada, Y., H. Bando, Y. Miyamoto, M. Kosaka and T. Sano. 1991. Nippon Naibunpi Gakkai Zasshi. 67:1162 -1177.
37. Vandeputte-Van Messom, G. and C. Burvenich. 1993. J. Dairy Sci. 76:3727-3741.
38. Veenhuizen, J.J., J.K. Drackley, M.J. Richard, T.P. Sanderson, L.D. Miller and J.W. Young. 1991. J. Dairy Sci. 74:4238-4253.
39. Vernon, R.G. 1989. Influence of somatotropin on metabolism. In: Use of Somatotropin in Livestock Production, Eds. K. Sejresen, M. Vestergaard and A. Neimann-Sorensen.Elsevier Applied Science, New York, NY, pp. 31 - 50.
40. Vicini, J.L., P.K. Olsson, B. Barnes and R.J. Collier. 1995. J. Dairy Sci. 78 (Suppl. 1):180.
41. Vicini, J.L., C.A. Baile, G.F. Hartnell and J.J. Veenhuizen. 1993. J. Dairy Sci. 76(Suppl. 1):276.
42.Vicini, J.L., F.C. Buonomo, J.J. Veenhuizen, M.A. Miller, D.R. Clemmons and R.J. Collier. 1991. J. Nutr. 121:1656-1664.
43. Waterman, D.F., W.J. Silvia, R.W. Hemken, G. Heersche, Jr., T.S. Swenson and R.G. Eggert. 1993. Theriogenology 40:1015-1028.
44. Webb, R., J.G. Gong and T.A. Bramley. 1994. Theriogenology 41:25-30.
45. White, T.C., K.S. Madsen, R.L. Hintz, R.H. Sorbet, R.J. Collier, D.L. Hard, G.F. Hartnell, W.A. Samuels, G. de Kerchove, F. Adriaens, N. Craven, D.E. Bauman, G. Bertrand, P. Bruneau, G.O. Gravert, H.H. Head, J.T. Huber, R.C. Lamb, A.N. Pell, R. Phipps, R. Weller, G. Piva, Y. Rijkema, J. Skarda, F. Vedeau and C. Wollny. 1994. J. Dairy Sci. 77:2249-2260.