Introduction
Generally, it has been assumed that endogenously produced ascorbic acid was sufficient to meet the metabolic demands of ruminants. However, recent research on swine and poultry (17, 23) suggests that under specific environmental and physiological conditions the amount of ascorbic acid produced by the animal may in fact be insufficient to meet its requirements. Lactating dairy cattle may be predisposed to subclinical ascorbic acid deficiency because of the huge drain on the precursor molecules for ascorbic acid production, glucose and galactose into milk. Non-lactating ruminants may also have their ascorbic acid status compromised during periods of stress (11). The physiological ramifications of subclinical ascorbic acid deficiency are multifactorial. Ascorbic acid is critical if the effectiveness of the immune system is to be optimized (4, 5). Pathogenic organisms such as bacteria and viruses cause increased free radical production in the infected host and a concomitant decrease in ascorbic acid level and immune function (3).Neutrophils are the primary leukocyte responsible for the killing of invading pathogens. A deficiency of ascorbic acid can reduce the ability of the cell to migrate to the site of the inflammation, allowing for increased oxidative damage to the neutrophils and reduced production of the major antimicrobial agent, hypochlorous acid. Hypochlorous acid can also exert a negative effect on lymphocyte proliferation. In the presence of adequate levels of ascorbic acid lymphocyte proliferation occurs normally (1). Ascorbic acid and other antioxidant vitamins such as vitamin E have been shown to enhance neutrophil function and minimize free radical damage (18).
Ascorbic acid may also modulate the immune system via its role in the regulation of hormones associated with stress. Acute stressors activate the hypothalamic-pituitary-adrenal axis resulting in the elaboration of corticotrophin releasing hormone (CRH). Corticotrophin releasing hormone stimulates the anterior pituitary to release adrenocorticotropic hormone which in turn causes the adrenal cortex to increase circulating plasma levels of glucocorticoids (20). Glucocorticoids, specifically cortisol, compromise many facets of the cellular and humoral immune response.
Vitamin C is thought to have a role in the production and regulation of corticosteroid production in the bovine adrenal gland (9). In addition, stress and the associated rise in corticosteroids may reduce the circulating plasma levels of ascorbic acid (17).
Vitamin C is a known antioxidant and in this capacity can quench free radicals and thereby protect the structural integrity of the cells of the immune system (6). The antioxidant properties of ascorbic acid may also impart improved quality to beef (25). Cattle fed high levels of another antioxidant vitamin, alpha-tocopherol, produced steaks that had superior shelf life in terms of color and lipid oxidation (2). There is a close synergism between ascorbic acid and vitamin E in that vitamin C can reduce the radical of vitamin E back to its active reduced state (15). Vitamin C, therefore, may play a two-fold role in preventing the oxidation of the meat color pigment, oxymyoglobin, to the less desirable metmyoglobin. Metmyoglobin is the pigment which causes fresh beef to become brown in color thus reducing its consumer appeal. Ascorbic acid may enhance the quality of beef directly by acting as antioxidant and indirectly by preserving the antioxidant potential of vitamin E.
Ascorbic acid has been shown to have interactions with trace minerals in species other than ruminants (10). In particular, there has been considerable research on the interaction between iron and ascorbic acid in swine (23). Intestinal absorption of nonheme iron is known to increase in the presence of ascorbic acid (23). Iron is transferred into mucosal cells in the ferrous form and because vitamin C can reduce ferric iron to ferrous iron it may facilitate absorption (24).
In contrast to iron, copper absorption may actually be reduced by ascorbic acid. Vitamin C can reduce the divalent copper ion to the less absorbable monovalent ion (24).
There has been far less research conducted on any interactions that may occur between zinc absorption and ascorbic acid. The studies that have been completed in this area are often contradictory in nature (21).
Ascorbic Acid, Rumen Stability
 In cattle that have been injected with synthetic glucocorticoids (dexamethasone) the negative effect
on the neutrophil function of dairy cows could be partially alleviated by the injection of ascorbic
acid (19). Unfortunately, injecting ascorbic acid can often lead to inflammation at the site of
injection (14). Therefore, there exists a need for a suitable form of vitamin C that could be used as
a feed supplement.
In cattle that have been injected with synthetic glucocorticoids (dexamethasone) the negative effect
on the neutrophil function of dairy cows could be partially alleviated by the injection of ascorbic
acid (19). Unfortunately, injecting ascorbic acid can often lead to inflammation at the site of
injection (14). Therefore, there exists a need for a suitable form of vitamin C that could be used as
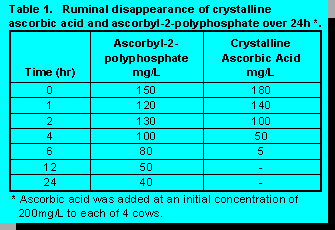
a feed supplement.Ascorbic acid in its crystalline state is extremely unstable in the conditions which exist in the rumen (12). Therefore, a new vitamin C product form, ascorbyl-2-polyphosphate was tested to determine rumen stability. Table 1 illustrates that the ascorbyl-2-polyphosphate did indeed have increased stability over the crystalline ascorbic acid. It can be concluded that ascorbyl-2-polyphosphate possesses sufficient rumen stability characteristics to warrant in vivo animal studies.
Ascorbyl-2-polyphosphate Supplementation
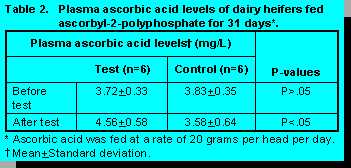
Ascorbyl-2-polyphosphate appears to be an ascorbic acid compound which has sufficient rumen stability to increase ascorbic acid plasma levels of dairy heifers fed the product for 31 days (Table 2). Prior to the commencement of the trial plasma ascorbic acid levels of the control and test groups were 3.83 and 3.72mg/L, respectively (P<0.05) (Table 2). The fact that the plasma levels remained elevated even after 31 days suggests that the animals did not compensate for the exogenous supply of ascorbic acid by reducing their endogenous production of the vitamin.
 Biopsies of the biceps femoris of heifers were performed at the beginning and end of the trial. The
reasons biopsies were required were: 1) an additional indication as to the bioavailability of the
ascorbyl-2-polyphosphate, and 2) to determine the possible use of this product formula to raise
meat ascorbic acid levels. Increased meat ascorbic acid may improve the quality of retail beef
(25).Initially there was no difference between the tissue ascorbic acid levels of the test and control
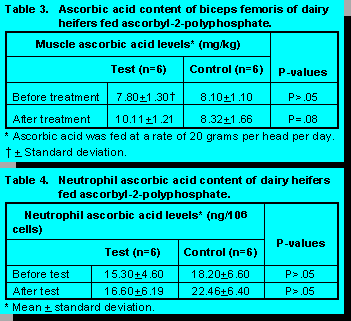
animals, 7.80 versus 8.20 mg/kg wet weight (P>0.05) (Table 3). After the treatment there was a
definite trend for the animals receiving ascorbic acid to have increased levels of muscle ascorbic
acid. The tissue levels were 10.11 and 8.32 mg/kg ascorbic acid for the test and control animals
(P=.08) (Table 3). The results of the plasma and muscle ascorbic acid analyses together support
the view that ascorbyl-2-polyphosphate is a suitable source of ascorbic acid for ruminants.
Biopsies of the biceps femoris of heifers were performed at the beginning and end of the trial. The
reasons biopsies were required were: 1) an additional indication as to the bioavailability of the
ascorbyl-2-polyphosphate, and 2) to determine the possible use of this product formula to raise
meat ascorbic acid levels. Increased meat ascorbic acid may improve the quality of retail beef
(25).Initially there was no difference between the tissue ascorbic acid levels of the test and control
animals, 7.80 versus 8.20 mg/kg wet weight (P>0.05) (Table 3). After the treatment there was a
definite trend for the animals receiving ascorbic acid to have increased levels of muscle ascorbic
acid. The tissue levels were 10.11 and 8.32 mg/kg ascorbic acid for the test and control animals
(P=.08) (Table 3). The results of the plasma and muscle ascorbic acid analyses together support
the view that ascorbyl-2-polyphosphate is a suitable source of ascorbic acid for ruminants.
Neutrophil function can be enhanced in humans by the supplementation of dietary ascorbic acid (6). Neutrophil ascorbic acid levels in humans are normally 24ng/106 cells (13) which mimics closely the levels reported in Table 4 for bovine neutrophils. The data were quite variable between animals and there were not any differences (P>0.05) between groups at any stage of the trial. There are several explanations for the enhanced plasma levels not translating into increased neutrophil ascorbic acid levels. Firstly, neutrophils actively transport ascorbic acid against a large concentration gradient, therefore, the cells can maintain a homeostatic level of the vitamin unless the host animal is deficient (22). It appears the trial animals were producing sufficient ascorbic acid to fulfil their metabolic requirements. Secondly, the amount of time needed to harvest and separate the cells may have been too long. Ascorbic acid is extremely labile and therefore, the time for processing may have masked any potential effects.
Ascorbic Acid Supplementation to Calves
Newborn calves are dependent upon external sources of ascorbic acid. The calf's inborn ascorbic acid production does not begin until 2 to 3 weeks of age and does not reach a maximum until 8 to 16 weeks (16).The ascorbic acid requirements of calves is dependent on the genetic predisposition of the individual animals to produce the compound (16). Requirements are also influenced by environment, for example extensive confinement can increase the metabolic demand for vitamin C (8).
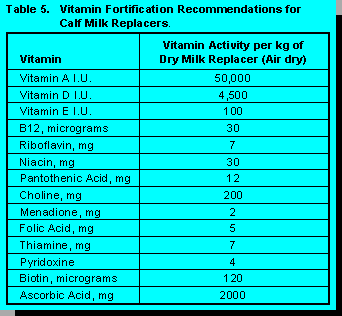
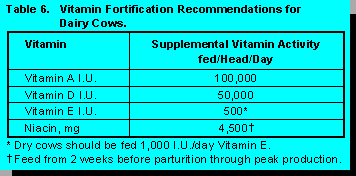
When calves are subjected to environmental stressors the supplementation of ascorbic acid has been shown to enhance the immune response of the calves (7). The suggested levels of ascorbic acid and all other pertinent vitamins in milk replacers are shown in Table 5. Vitamin recommendations for dairy cows are shown in Table 6.
Conclusion
The most rumen stable form of ascorbic acid found to date is ascorbyl-2-polyphosphate. Ascorbic acid in the polyphosphate form now makes it possible to conduct large scale production studies to determine what effect the vitamin will have on the productivity and profitability of dairy herds.It can be generally recommended that milk replacers should contain supplemental ascorbic acid.
Additional Readings
Applied Dairy Science Course - University of Alberta:Nutrient Requirements of Dairy Cattle
References
1. Anderson, R., M.J. Smit, G.K. Joone and A.M. Van Staden. 1990. Vitamin C and cellular immune functions: Protection against hypochlorus acid-mediated inactivation of glyceraldehyde-3-phosphate dehydrogenase and ATP generation in human leukocytes as a possible mechanism of ascorbate mediated immunostimulation In: Micro Nutrients and Immune Functions (Eds A. Bendich and R.K. Chandra) New York, U.S.A.2. Arnold, R.N., K.K. Scheller, S.C. Arp, S.N. Williams, D.R. Buege and Schaefer, D.M. 1992. Effect of long or short term feeding of -tocopherol acetate to Holstein and crossbred beef steers on performance, carcass characteristics and beef color stability. J. Anim. Sci. 70:3055-3065.
3. Beisel, W.R. 1982. Single nutrients and immunity. Am. J. Clin. Nutr. 35:417-418.
4. Bendich, A. 1989. Antioxidant vitamins and their functions in immune responses. In: Antioxidant Nutrients and Their Immune Functions (Eds A. Bendich, M. Phillips and R.B. Tengerdy) Plenum Publishing Co. New York, U.S.A. pp. 408-421.
5. Bendich, A. 1992. Ascorbic acid and immune functions. In: Ascorbic Acid in Domestic Animals (Eds C. Wenk, R. Fenster and L. Volkerl) Institut fur Nutzlierwissenschaften, Zurich, Switzerland.
6. Bendich, A., L.J. Machlin, O. Scandurra, G.W. Burton and D.M. Wayner. 1986. The antioxidant role of vitamin C. Free Rad. Biol. Med. 2:419-444.
7. Blair, L. and K. Cummins 1984.Effect of dietary ascorbic acid on blood immunoglobulins concentration in dairy cows. J. Dairy Sci. 67:138-139.
8. Cummins, K.A. and C.J. Brunner. 1989. Dietary ascorbic acid and the immune response in dairy calves. J. Dairy Sci. 129-134.
9. Finn, F.M. and P.A. Johns. 1980. Ascorbic acid transport by isolated bovine adrenal cortical cells. Endocrinology 106:811-817.
10. Hilton, J.W. 1984. Ascorbic acid-mineral interactions in fish. In: Ascorbic Acid in Domestic Animals (Eds I. Wegger, F.J. Jagwerker and J. Moustguard) Royal Danish Agricultural Society, Copenhagen, Denmark.
11. Hirdiroglou, M., M. Ivan, and J.R. Lessar. 1977. Effect of ration and inside versus outside housing on plasma levels of ascorbic acid, lactic acid, glucose and cholesterol in hereford steers wintered under practical conditions. Can. J. Anim. Sci. 57:519-529.
12. Knight, C.A., R.A. Dutcher, N.B. Geurrent and S.J. Bechdel. 1941. Utilization and excretion of ascorbic acid by the dairy cow. J. Dairy Sci. 24:567-577.
13. Kutnink, M.A., W.C. Hawkes, E.E. Schaus and S.T. Omgye. 1987. An internal standard method for the unattended high performance liquid chromatographic analysis of ascorbic acid in blood components. Anal. Biochem. 166:424-430.
14. Loscher, W., G. Jaeschke and H. Keller. 1984. Pharmacokinetics of ascorbic acid in horses. Equine vet J. 16:59-65.
15. Niki, E., T. Saito, A. Kawakami and Y. Kamiya. 1984. Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. Biol. Chem. 259:4177-4182.
16. Palludan, B. and I. Wegger. 1984. Plasma ascorbic acid in calves. In: Ascorbic Acid in Domestic Animals. (Eds. I. Wegger, F. Tagwerker and J. Moustgaard) Royal Danish Agricultural Society, Copenhagen, Denmark. pp. 131-138.
17. Pardue, S.L., J.P. Thaxton and J.J. Brake. 1985. Role of ascorbic acid in chicks exposed to high environmental temperature. Appl. Physiol. 58:1511-1516.
18. Politis, I., M. Hidiroglou, R.T. Batra, J.A. Gilmore, R.C. Gorewit and H. Sherf. 1995. Effects of vitamin E on immune function of dairy cows. Am. J. Vet. Res. 56:179-184.
19. Roth, J.A and M.L. Kaeberle. 1985. In vivo effect of ascorbic acid on neutrophil function in healthy and dexamethasone treated cattle. Am. J. Vet. Res. 46:24-36.
20. Scott, G.H. 1981. What is animal stress and how is it measured. J. Anim. Sci. 52:150-153.
21. Solomons, N.W. and F.E. Viteri. 1982. Biological interactions of ascorbic acid and mineral nutrients. Adv. Chem. Series 200:551-569.
22. Washko, P., D. Rotrosen and M. Levine. 1989. Ascorbic acid transport and accumulation in human neutrophils. J. Biol. Chem. 264:8996-9002.
23. Yen, J.T. and W.G. Pond. 1981. Effect of dietary vitamin C addition on performance, plasma vitamin C and hematic iron status in weanling pigs. J. Anim. Sci. 53:1292-1296.
24. Yen, J.T. and W.G. Pond. 1984. Ascorbic acid interaction with iron, copper, selenium and vitamin E. In: Ascorbic Acid in Domestic Animals (Eds. I. Wegger, F.J. Tagwerker and J. Moustgaurd). Royal Danish Agricultural Society, Copenhagen, Denmark. pp.542-549.
25. Yin, M.C., C. Faustman, J.W. Riesen and S.N. Williams. 1993.-Tocopherol and ascorbate delay oxymyoglobin and phospholipid oxidation in vitro. Food Sci. 6:1273-1276.