Introduction
Good nutritional management and adequate intakes of energy substrates, water, protein, fat, minerals, and vitamins are important for growth, milk production, and reproduction of the dairy cow. The key roles of the reticulo-rumen (RR) and its resident microorganisms in diet utilization and synthesis of essential nutrients are well known. The microorganisms present in the rumen use the nutrients supplied by the animal for growth. In doing so they release energy from dietary fibre for microbial protein synthesis. The extent of microbial protein synthesis and the products of ruminal digestion vary depending on the diet of the animal. Differences in rates of degradation of forage and concentrates ensure a diurnal variation in nutrient supply to cows. In addition, the process of digestion in the small intestine also ensures that the amount and composition of absorbed nutrients vary throughout the day. The process of digestion and the absorption of specific nutrients activate complex neural and endocrine circuits that influence gene expression in the gut, in the peripheral tissues, and in the brain. Genes are activated in response to both undersupply and oversupply of specific nutrients by means of feedback mechanisms. The complexities of internal, metabolic and environmental cues require an integration role for the central nervous system. However, the mechanism by which each nutrient (e.g., volatile fatty acid, amino acid, or glucose) is detected and turns on specific genes are not well known. This review deals with the potential nutrient-gene interactions and details how nutrients affect genetic activity to enhance productivity of dairy cows. The review is divided into sections that deal with regulatory hormones of the gut, the transport of nutrients across the gut, and the effect of nutrients on the hormones that modulate the processes of growth and productivity of the dairy cow.
Nutrient Control of Gastrointestinal Hormone Gene Expression
The gastrointestinal tract (GIT) hormones, also known as regulatory peptides, are a large and complex group of peptides synthesized by cells of a diffuse and heterogenous endocrine system in the mucosal lining of the GIT (19). They are responsible for the optimal digestion and absorption of nutrients by controlling and influencing the secretion of the necessary electrolytes and enzymes, and gastrointestinal motility. The actions of the GIT peptide hormones including stimulation and reduction of secretions, motility, and absorption are regulated by specific nutrients (18). However, until recently not much was known about whether the effects of the specific nutrients on GIT hormones were mediated by, linked to, or influenced by GIT hormone gene expression. Progress in this area is discussed with emphasis on gastrin, cholecystokinin (CCK), and somatostatin plus an overview of secretin, enteroglucagon, pancreatic polypeptide (PP), and vasoactive-intestinal polypeptide (VIP).
Nutrient Control of Gastric Gene Expression
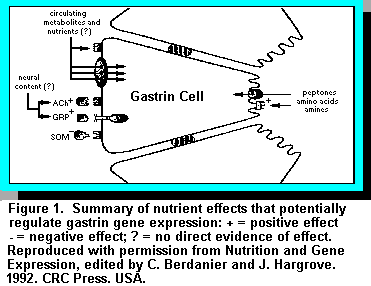 Gastrin, which exists as two major forms of 34 (G34) and 17 (G17) amino acids, is
synthesized in substantial amounts in the G cells of the antrum of the abomasum and duodenum
and is present in higher concentrations in the antrum (18, 19). The two forms potentially are
derived from a differential processing of a single precursor encoded by a single gene and mRNA
(19). Gastrin serves two major functions, the stimulation of abomasal acid secretion and
stimulation of mucosal growth in the acid-secreting portion of the abomasum. However, the effect
of gastrin on acid secretion in cattle is poorly understood (18). It is now known that restricted
feeding is associated with decreases in gastrin concentrations in the antrum and in antral G cell
density and refeeding reverses these effects (13). Indeed, it is now well accepted that the chemical
composition of feedstuffs (i.e., protein content) and the digestive products of proteins (i.e.,
cysteine, phenylalanine, tryptophan, and calcium) are potent stimulants of gastrin release (7).
However, varying the protein contents of diets does not affect circulating gastrin levels in
ruminants (12). Recent data show that the effects of fasting and specific nutrient-associated
changes in circulating and antral gastrin concentrations are mediated at the level of gastrin mRNA
(Figure 1).
Gastrin, which exists as two major forms of 34 (G34) and 17 (G17) amino acids, is
synthesized in substantial amounts in the G cells of the antrum of the abomasum and duodenum
and is present in higher concentrations in the antrum (18, 19). The two forms potentially are
derived from a differential processing of a single precursor encoded by a single gene and mRNA
(19). Gastrin serves two major functions, the stimulation of abomasal acid secretion and
stimulation of mucosal growth in the acid-secreting portion of the abomasum. However, the effect
of gastrin on acid secretion in cattle is poorly understood (18). It is now known that restricted
feeding is associated with decreases in gastrin concentrations in the antrum and in antral G cell
density and refeeding reverses these effects (13). Indeed, it is now well accepted that the chemical
composition of feedstuffs (i.e., protein content) and the digestive products of proteins (i.e.,
cysteine, phenylalanine, tryptophan, and calcium) are potent stimulants of gastrin release (7).
However, varying the protein contents of diets does not affect circulating gastrin levels in
ruminants (12). Recent data show that the effects of fasting and specific nutrient-associated
changes in circulating and antral gastrin concentrations are mediated at the level of gastrin mRNA
(Figure 1).Feeding causes a rapid increase in gastrin mRNA and effects can be apparent in as little as 15 minutes, especially if the feed contains specific nutrients such as amino acids and fats. The rapidity of such a response suggests that nutrient effects are on gastrin gene transcription or mRNA stability (Figure 1).
Nutrient Control of Cholecystokinin (CCK) Gene Expression
Cholecystokinin (CCK) is closely related to gastrin, but it is the product of a different gene. CCK is secreted by cells of the proximal small intestine and has long been recognized as a potent stimulant of pancreatic enzyme and gallbladder contraction and an inhibitor of gastric emptying (14). In cattle, CCK is considered to be one of the major hormones released in the intestinal phase of digestion. The effects of nutrition on CCK release in cattle is not well documented. For example, it is not known what causes CCK to be released from the intestine of ruminants although intensive research efforts are underway in our laboratory (17) and elsewhere. Intravenous injection of CCK and intra duodenal injection of a trypsin inhibitor such as soybean extracts, stimulates CCK release, enhances pancreatic flow rate, and enzyme output in calves (20). In other species fats, fatty acids, protein, and amino acids are potent stimulants of CCK secretion, whereas glucose is a less important stimulator. It is known that CCK secretion is controlled by a feedback mechanism and is stimulated by inactivation of intestinal proteases or by binding of proteases to a trypsin inhibitor (4). Iwai and co-workers (4) have hypothesized that the rate of CCK release is linked to the amount of protein within the duodenal lumen via a new CCK releasing peptide called monitor peptide. Monitor peptide possesses trypsin inhibitor activity and competes with proteins in the duodenum for binding to trypsin. In the presence of adequate protein, monitor peptide remains free from trypsin and stimulates CCK secretion from duodenal mucosa (Figure 2). It is not known whether a similar monitor peptide exists in cattle. However, depriving animals of feed produces a gradual and progressive decline in CCK mRNA.
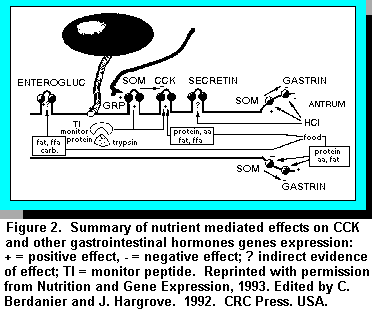 The absence of nutrients may influence CCK gene transcription, mRNA stability, or abundance of
CCK cells in comparison to other cell types in the duodenum (8). Refeeding restores CCK mRNA
levels to normal within one day and there is increasing evidence that direct stimulatory effects of
protein or other nutrients on CCK secreting cells play an important part in the control of feed
intake and productivity of the cow (Figure 2). For example we believe that for the pancreas to
release starch digesting enzymes it needs to be stimulated by CCK which in turn is inhibited by
trypsin. A high protein content in the diet ensures that the monitor peptide is less likely to bind to
trypsin. This increases CCK secretion, thereby stimulating amylase release and starch digestion in
the small intestine (10).
The absence of nutrients may influence CCK gene transcription, mRNA stability, or abundance of
CCK cells in comparison to other cell types in the duodenum (8). Refeeding restores CCK mRNA
levels to normal within one day and there is increasing evidence that direct stimulatory effects of
protein or other nutrients on CCK secreting cells play an important part in the control of feed
intake and productivity of the cow (Figure 2). For example we believe that for the pancreas to
release starch digesting enzymes it needs to be stimulated by CCK which in turn is inhibited by
trypsin. A high protein content in the diet ensures that the monitor peptide is less likely to bind to
trypsin. This increases CCK secretion, thereby stimulating amylase release and starch digestion in
the small intestine (10).
Nutrient Control of Somatostatin Gene Expression
Originally isolated from sheep hypothalami, somatostatin is now known to be present in two forms. The 14 amino acid somatostatin (S-14) exerts its effect on the brain and the 28 amino acid somatostatin (S-28) principally in the small intestine (18). In general, somatostatin acts to inhibit gastric acid secretion, pancreatic exocrine secretion, and secretion of hormones from the pancreas and GIT endocrine cells (Figures 1 and 2). Acidification of the contents of the antrum of the abomasum and duodenum stimulate somatostatin release. Immunization against somatostatin has been tested as a means of increasing growth in ruminants. Within 12 hours of feed deprivation, somatostatin mRNA levels increase in the gastric antrum with no effect on duodenal mRNA levels. These results suggest that feed deprivation induces region-specific effects on somatostatin gene transcription and/or mRNA stability within the GIT (5). Increased feeding levels result in a rapid decline in gastric antrum levels of somatostatin mRNA without much effect on duodenal mRNA levels. The details of the mechanisms are still not well known.
Glucose Transport and Metabolism
Nutrients need to be transported across the gut into the blood stream and into cells before they can be used for productive purposes by the dairy cow. The transport systems of the gut are extremely complex. Transport of nutrients is not a random process, but rather a well-controlled system that responds to both supply of nutrients to the gut and the nutrient needs of the animal. In this section we will discuss the transport of glucose as an example of the manner in which the gut controls and directs the supply of nutrients to the animal. In recent years many advances have been made in the structural identification of intestinal glucose transporters in ruminants and the molecular mechanisms controlling the synthesis of the glucose transporter in response to signals initiated by dietary changes. The upper limit on optimal starch digestion in the small intestine espoused by Kreikemeier et al. (6) among others have been interpreted to mean the capacity of glucose transporters is exceeded even if there is adequate starch digestion in the rumen (3). The reader should refer to Okine and Kennelly (10) and Okine et al. (11) on work detailing transport of glucose in lactating cattle.Studies of sheep and cattle representative of production situations indicate that 30 to 40% of starch digested in the small intestine appears as glucose in the portal vein of the liver (6). These results show that glucose is digested, absorbed, and transported into the portal vein. Transport of luminal glucose is by the Na+ -dependent D-glucose cotransporter (SGLT1) located on the brush border of cells of the small intestine. The sheep intestinal SGLT1 consists of 664 amino acids and exhibits a homology of 85% identity and 93% similarity to the rabbit sequence. Work done by Shirazi-Beechey and co-workers (15, 16) show that infusing glucose into the lumen of sheep through a duodenal cannula increased the rate of SGLT1 transporter levels. The diet induced changes of SGLT1 activity were proportional to SGLT1 protein abundance, but were not matched by major changes in SGLT1 mRNA levels. Shirazi-Beechey et al. (15, 16) have also reported that the levels of SGLT1 decline if animals are fed high-forage diets because glucose is necessary to induce SGLT1 expression. It should be noted that infusion of starch into the intestine of sheep for four days did not lead to the induction of SGLT1 (Shirazi-Beechey et al., unpublished data ). We have now shown in our laboratory that SGLT1 mRNA is expressed when cows are fed high levels of a barley-based concentrate in early-lactation. Experiments to show the modulation of both the mRNA and protein of SGLT1 when cows are fed different starch sources are now in progress in our laboratory.
The 60 to 70% of digested glucose is partitioned between the cells of the small intestine, pancreas, spleen, mesentery, and omentum plus the mammary gland. Okine et al. (10) showed that in vitro, more than 50% of glucose absorbed by cells of the small intestine is metabolized to carbon dioxide. We (11) have also shown that unlike monogastric animals, cells of the small intestine of ruminants seem to prefer glucose to glutamine (an amino acid) as an energy source. The tissues (small intestine, pancreas, spleen, mesentery, and omentum) all have very high metabolic rates and their high glucose usage reflects the high turnover and operating costs of nutrient digestion and absorption (3).
As the milk production potential of dairy cows has increased, the requirement for glucose to meet the needs of various tissues has also increased. Thus, adding increased levels of cereal grains to meet the energy needs of these animals has been necessary. When animals are fed high grain diets, glucose synthesis follows a similar pattern to that observed on forage-based diets, however, there are some important differences.
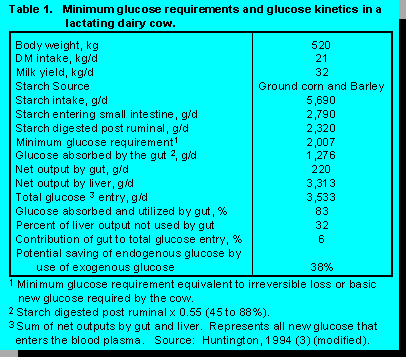
The point to be made from the table is that gut metabolism of glucose from the luminal side saves about 32% of endogenously produced glucose by the liver thus allowing more glucose to be directed to the mammary gland for milk synthesis.
Nutritional Regulation of Anabolic Hormones
Nutrient Regulation of Insulin Gene Expression
 The major pancreatic islet hormones, insulin and glucagon, are rapid and
powerful regulators of metabolism. They regulate and coordinate the disposition of nutrient input
from meals and endogenous substances by actions on the liver, adipose tissue, and muscle mass.
Insulin is a peptide hormone secreted by the -cells of the pancreatic islets. In the broadest sense,
insulin secretion is governed by a feedback relationship with the exogenous nutrient supply. When
nutrient supply is high or abundant, insulin is secreted in response and the hormone in turn
stimulates the utilization of these nutrients while inhibiting the mobilization of endogenous
substrates. When nutrient supply is low, insulin secretion is dampened and there is an enhanced
mobilization of endogenous substrates. Short-term control of insulin production is regulated
through translation of preexisting mRNA. Over the longer term, insulin mRNA levels are
regulated through effects on the rate of transcription of the insulin gene, and mRNA stability (2).
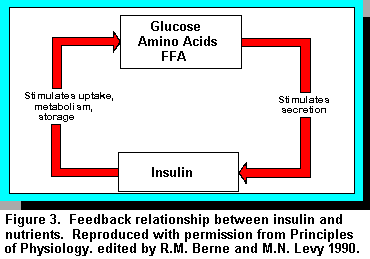
Insulin mRNA level is modulated by glucose through effects both on transcription of the insulin
gene and on the rate of turnover of insulin mRNA. Other nutrient substances that influence insulin
gene levels are mannose, amino acids, fatty acids/keto acids (Figure 3).
The major pancreatic islet hormones, insulin and glucagon, are rapid and
powerful regulators of metabolism. They regulate and coordinate the disposition of nutrient input
from meals and endogenous substances by actions on the liver, adipose tissue, and muscle mass.
Insulin is a peptide hormone secreted by the -cells of the pancreatic islets. In the broadest sense,
insulin secretion is governed by a feedback relationship with the exogenous nutrient supply. When
nutrient supply is high or abundant, insulin is secreted in response and the hormone in turn
stimulates the utilization of these nutrients while inhibiting the mobilization of endogenous
substrates. When nutrient supply is low, insulin secretion is dampened and there is an enhanced
mobilization of endogenous substrates. Short-term control of insulin production is regulated
through translation of preexisting mRNA. Over the longer term, insulin mRNA levels are
regulated through effects on the rate of transcription of the insulin gene, and mRNA stability (2).
Insulin mRNA level is modulated by glucose through effects both on transcription of the insulin
gene and on the rate of turnover of insulin mRNA. Other nutrient substances that influence insulin
gene levels are mannose, amino acids, fatty acids/keto acids (Figure 3).
Although short-term (20 minutes to a few hours) nutrient regulation of insulin secretion occurs at the level of translation of existing mRNA, long term regulation is mediated through changes in insulin mRNA. In this way, the -cells can rapidly replenish insulin stores, and enable them to respond to changes in the blood glucose level throughout the day while also having the capacity to adapt to more long-term dietary changes. The overall thrust of insulin is to facilitate storage of nutrients and inhibit their release. For carbohydrates, insulin stimulates the transport of glucose from the plasma, across the cell membrane, and into the cytoplasm in muscle and fat (adipose) tissue. For fats, insulin stimulates transfer of fatty acids into the adipose cells for fat synthesis. For protein, insulin stimulates the transport of certain amino acids from the plasma across the cell membrane for protein synthesis. These effects are summarised in Figure 4.
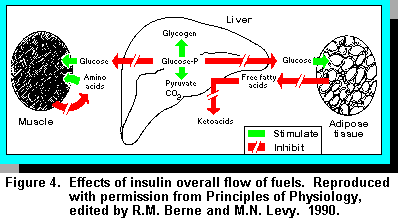
The overall synthesis of proteins from amino acids is also increased by stimulation of transcription and translation. These anabolic (buildup) effects of insulin are reinforced by the anticatabolic (anti-breakdown) effects, that is the inhibition of the enzymes of proteolysis and inhibition of the release of amino acids from the cell. In addition, insulin and the structurally related peptides called somatomedins enhance the general synthesis of proteins, DNA, RNA, and other macromolecules. Thus, insulin is an important contributor to growth, tissue regeneration, and increased productivity of the dairy cow.
Nutrient Regulation of Bovine Growth Hormone Gene Expression
Bovine growth hormone (bGH) is produced by the anterior pituitary gland and belongs to a family of somatolactogenic hormones including prolactin and placental lactogen. It has been known for a long time that exogenous bGH administration is galactopoietic, increases lipolysis, protein accretion, bone growth, gluconeogenesis, and improves the biological efficiency of milk production when injected into dairy cows (1). The current theory of the mechanism of action of bGH is that it exerts a chronic homeorhetic regulation of metabolism by repartitioning nutrients in favor of milk production. The natural secretion of endogenous GH is controlled by several factors including growth hormone releasing factor (GRF) which is stimulatory and somatostatin (SS) which is inhibitory. Like all peptide hormones, the first step in the action of GH is the binding of GH to receptors in target tissues. Although GH receptors have been found in various tissues, not much is known about the bGH receptor. However, it is known that two types exist, one receptor of high affinity and the other of low affinity. Although a direct effect on bGH on target tissues such as the mammary gland cannot be ruled out it is generally believed that the effects of bGH require the generation of a distinct class of second messengers called insulin-like growth factors (IGF) which modulate its intracellular actions.
The effects of plane of nutrition and specific nutrients on endogenous and exogenous bGH are both complex and interesting. Endogenous bGH is regulated metabolically by the energy substrates glucose and free fatty acids. A sharp drop in either glucose or free fatty acid levels stimulate a two- to ten-fold increase in plasma bGH, whereas elevation of glucose or free fatty acid levels reduces bGH levels by 60%. Protein ingestion, on the other hand, stimulates bGH synthesis.Detection of high-affinity GH receptors of insulin-like growth factor ( IGF-1) in response to rbGH injection in the dairy cow depends on the plane of nutrition. Dairy cows on a high plane of nutrition that respond to rbGH with increased IGF-1 concentrations also possess high affinity GH receptors in the liver and adipose tissues (1). However, cows fed diets deficient in energy and/or protein have little response in IGF-1 concentrations or high affinity GH receptors. It is now known that a low plane of energy and/or inadequate protein diet can uncouple the relationship between bGH and IGF-1, thereby causing cows to have no milk production response when injected with rbGH. Thus, although voluntary feed intake does not increase until several weeks after the initiation of rbGH injections and increased milk production, the maintenance of increased milk production is dependent on a high plane of nutrition for the cows.
The mechanisms of action of rbGH are to argument the availability of milk precursors in the blood, an increased synthetic ability of the mammary gland, a slowing down of the involution of the mammary gland and chronic lipolytic and nutrient partitioning to ensure increased milk production by the mammary gland. These mechanisms are possible and increased milk production probable only if the dairy cow is maintained on a high plane of nutrition.
References
1. Burton, J.L, B.W. McBride, E. Block, D.R. Glimm and J.J. Kennelly. 1994. A review of bovine growth hormone. Can J. Anim. Sci. 74:167.2. Docherty, K. and A.R. Clark. 1994. Nutrient regulation of insulin gene expression. FASEB J. 8:20.
3. Huntington, G.B. 1994. Ruminant starch utilization progress has been extensive. Page 16 in Feedstuffs (June 6).
4. Iwai, K., T. Fushiki and S.-I. Fukuoka. 1988. Pancreatic enzyme secretion mediated by a novel peptide: monitor peptide hypothesis. Pancreas 3:720.
5. Kanayama, S. and R.A. Liddle. 1991. Influence of food deprivation on intestinal Cholecystokinin and somatostatin. Gastroenterology 100:909.
6. Kreikemeier, K.K ., D.L. Harmon, R.T. Brant Jr., T.B. Avery and D.E. Johnson. 1991. Small intestinal starch digestion in steers: Effects of various levels of abomasal glucose, corn starch and corn dextrin infusion on small intestinal disappearance and net glucose absorption. J. Anim. Sci. 69:328.
7. Lichtenberger, L.M. 1982. Importance of food in the regulation of gastrin release and formation. Am. J. Physiol.. 243:G429.
8. Lund, P.K. 1993 Nutritional control of gastrointestinal hormone gene expression. In: Nutrition and Gene Expression, edited by C. Berdanier and J. Hargrove. 1993. CRC Press. USA. pp 91.
9. Nocek, J.E. and S. Tamminga. 1991. Site of digestion of starch in the gastrointestinal tract of dairy cows and its effect on milk yield and composition. J. Dairy Sci. 74:3598.
10. Okine, E.K and J.J. Kennelly. 1994. From Fiber to starch: the evolution of the cow. In: Advances in Dairy Technology. 1994. J. J. Kennelly (ed). Volume 6: 187.
11. Okine, E.K., D.R. Glimm, J.R. Thompson and J.J. Kennelly. 1995. Influence of stage of lactation on glucose and glutamine metabolism in isolated enterocytes from dairy cattle. Metabolism. 44:325.
12. Perry, K.W., T.E.C. Weekes, J.A. Rooke, D.S. Parker and D.G. Armstrong. 1988. Effect of protein intake on gastrin secretion in ruminants. In: Physiology of Digestion in the Ruminant. R.W. Dougherty (ed.) Butterworths, Washington, DC pp. 97.
13. Reynolds, G.W. and D.A. Titchen. 1977. Inhibition of gastric acid and pepsin secretion in sheep on first eating. Proc. Int. Union. Physio. Sci. 13:186P.
14. Reynolds G.W. and L.M. Mcleay. 1980. Secretin and Cholecystokinin activities in extracts of sheep intestine. Vet. Sci. Commun. 4: 53..
15. Shirazi-Beechey, S.P., B.A. Hirayama, Y. Wang, D. Scott, M.W. Smith and E.M. Wright. 1991. Ontogenic development of lamb intestinal sodium-glucose cotransporter is regulated by diet. J. Physiol.. 437:699.
16. Shirazi-Beechey, S.P., I.S. Wood, J. Dyer, D. Scott and T.P. King. 1995. Intestinal sugar transport in ruminants. In: Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction 1995. W.V. Engelhardt, S. Leonhard Marek, G. Breves, and D. Giesecke (eds). Ferdinand Enke Verlag Stuttgard. pp 117.
17. Suominen, A.H., D.R. Glimm and J.J. Kennelly. 1995. Cholecystokinin gene is expressed in bovine duodenal tissue. Ann. Zootech. 44:suppl. 283.
18. Titchen, D.A. 1986. Gastrointestinal peptide hormone distribution, release, and action in ruminants. In: L.P. Milligan, W.L. Grovum and A. Dobson (eds). Control of Digestion and Metabolism in Ruminants. Prentice-Hall, Englewood Cliff, N.J. pp 227.
19. Walsh, J.H. 1987. Gastrointestinal hormones In: Physiology of the Gastrointestinal Tract. L.R. Johnson (ed). Raven Press, New York. pp. 181.
20. Zabielski, R., P. Podgurniak, S.G. Pierzynowski and W. Barej. 1989. Stimulation of exocrine pancreas secretion by exogenous CCK-8 and secretin boluses during thermical vagal blockage. Asian-Aust. J. Anim. Sci. 2:117.