Introduction
Protein is one of the major limiting nutrients in the diets of lactating dairy cows. This is particularly the case for cows in early lactation when DM intake is relatively low and protein requirement is high. Feeding a diet containing more protein is not a satisfactory solution because the breakdown of dietary protein in the rumen is one of the most inefficient processes in ruminant nutrition.In typical Alberta dairy rations, only 25 to 35% of the feed protein reaches the small intestine for absorption. In an attempt to overcome this inefficiency, dietary protein sources that are considered to be good sources of "bypass" or rumen undegradable intake protein (UIP) have been used.An alternate strategy is to feed rumen-protected amino acids (RPAA) so that any AA imbalances are corrected and overall utilization of dietary protein is improved. The purpose of this paper is to demonstrate the potential of RPAA in diet formulation. Further, information will be provided so that dairy producers can select an RPAA product that will be effective and economical.
Requirement for Amino Acids
Animals do not actually have a requirement for protein. Instead, they require the specific AA that are the building blocks that make up proteins. Therefore, the limiting factor in most dairy rations is the first or most limiting AA. It is generally accepted that methionine and lysine are most often the first limiting AA for milk production (28), although other AA have been suggested as first limiting (17). Cows also require glucose or its precursors for lactose synthesis and for glycerol, which is the "backbone" of milk fat. While AA are major sources of energy and glucose precursors, these needs can generally be met more economically from dietary starch and end products of ruminal digestion (volatile fatty acids; VFA).Ruminants obtain AA from microbial protein and from UIP. Rumen microbial protein supplies over half of the AA absorbed by ruminants. Microbial protein is a relatively well balanced protein source that has an essential AA profile that is similar to what cattle require for growth and milk production (Table 1). Therefore, rumen microbes are an excellent source of high quality protein relative to most feed proteins. Rumen protozoa are higher in lysine and lower in methionine than bacteria, but the presence of protozoa does not affect the AA profile of protein flowing from the rumen (18). This indicates that protozoa contribute little to the quality of protein flowing from the rumen. While it is a well balanced source of protein, production of microbial protein is limited by the fermentability of the diet and the amount of rumen degradable intake protein (DIP) in the diet. Therefore, microbial protein alone is insufficient to meet the requirements for high levels of milk production.
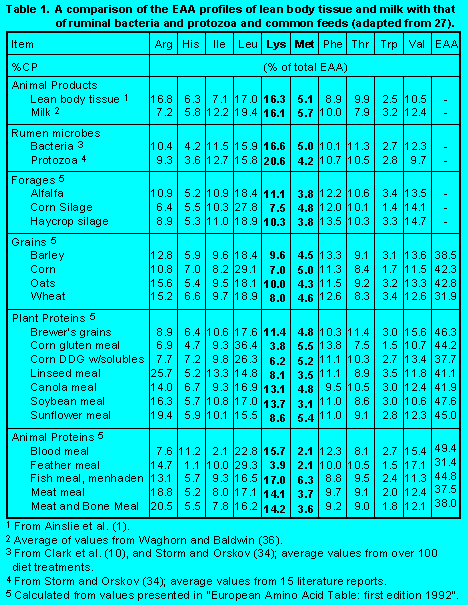
In recent years, productive diets for ruminants have been supplemented with various sources of UIP. Of the more common sources, fishmeal, meat and bone meal, feather meal, and corn gluten meal have been used. Based on AA profiles and rumen degradability, corn and its by-products (e.g. corn gluten meal) are relatively good sources of leucine, but are low in lysine. Fishmeal is a good source of methionine, but soybean meal is not. Blood meal is a good source of lysine, but is low in methionine. Feather meal is high in branched-chain AA. Thus, it is obvious that there is no one perfect source of AA.
Heat treatment has been used to decrease ruminal degradation of proteins and AA. Heating causes carbonyl groups of sugars to combine with free amino groups of proteins in the Maillard reaction. Amino acids also form peptide links with asparagine and glutamine (4). The resulting peptide linkages from heating are more resistant to enzymatic hydrolysis. Oilseed protein sources are the most economical to treat with heat. For example, Benchaar et al. (6) reported that feeding extruded lupins increased the flow of AA in the small intestine by 58%. Roasting and extrusion have also been extremely popular methods to increase the UIP content of soybeans. Some precautions must be taken when heat-treating proteins, as excessive heat can reduce the availability of essential AA such as lysine, methionine, and cystine.
 Increasing the amount of UIP has not always increased the amount or changed the quality of AA
reaching the small intestine. In some instances microbial protein production has decreased when
UIP increased, probably because of a reduction in diet fermentability. This caused an increase in
UIP, but a decrease in microbial protein, resulting in no net change in total AA flow to the small
intestine. No single feed source of UIP provides a balance of essential AA that matches the
essential AA profile of milk. In addition, many feeds with high UIP values are low in one or more
essential AA. As a result, a deficiency of one AA could be exacerbated by feeding a UIP source
low in that AA. Combinations of several UIP proteins that are complementary to each other could
overcome this problem. Ferguson et al. (11) reported on a research study involving 35 herds and
7,000 cows. Cows were supplemented with a marine-animal protein blend to attain a similar
protein level as unsupplemented cows. Nineteen of the thirty-five herds had a positive response
(79% of cows) where cows averaged 1.2 kg more milk per cow per day. In early lactation cows
only, 26 of 35 herds responded (95% of cows) with an average increase of 2.6 kg more milk per
day.
Increasing the amount of UIP has not always increased the amount or changed the quality of AA
reaching the small intestine. In some instances microbial protein production has decreased when
UIP increased, probably because of a reduction in diet fermentability. This caused an increase in
UIP, but a decrease in microbial protein, resulting in no net change in total AA flow to the small
intestine. No single feed source of UIP provides a balance of essential AA that matches the
essential AA profile of milk. In addition, many feeds with high UIP values are low in one or more
essential AA. As a result, a deficiency of one AA could be exacerbated by feeding a UIP source
low in that AA. Combinations of several UIP proteins that are complementary to each other could
overcome this problem. Ferguson et al. (11) reported on a research study involving 35 herds and
7,000 cows. Cows were supplemented with a marine-animal protein blend to attain a similar
protein level as unsupplemented cows. Nineteen of the thirty-five herds had a positive response
(79% of cows) where cows averaged 1.2 kg more milk per cow per day. In early lactation cows
only, 26 of 35 herds responded (95% of cows) with an average increase of 2.6 kg more milk per
day.
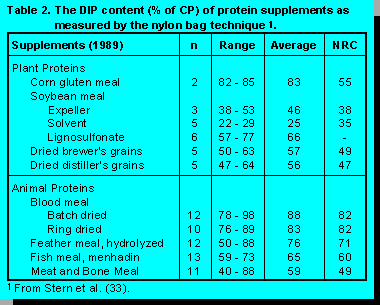
Responses to supplemental UIP are not always positive. Theurer et al. (35) summarized 88 experiments where high-UIP protein supplements replaced soybean meal. Milk yield increased in 17 experiments and milk protein percentage increased in only five experiments. Our inability to predict production responses to supplemental UIP are due to a number of factors. There is no accepted standard method to measure the UIP content of feedstuffs. When we change protein sources, we change DIP as well as UIP content of the diet. As stated above, this will affect rumen fermentation and consequently, the amount of microbial protein production. While the differences in UIP content is recognized among feedstuffs, the extreme within-feedstuff variability is seldom considered (Table 2). In addition, dietary factors that affect microbial access to the feed (e.g., feed particle size) and rumen environment (e.g., turnover rate, pH, and proteolytic activity) will alter the UIP content of feedstuffs.
Animal Response to Amino Acid Supplementation
Numerous studies have been conducted where combinations of AA and casein have been infused into the small intestines of dairy cows (26, 28, 29, 30). Most of these studies have used diets that are typical of those used on North American dairy farms and responses should be indicative of what can be expected at the farm level. Casein infusion typically results in cows producing more milk volume and milk protein, although the effect on milk protein percentage is variable. Milk fat content is unaffected by casein infusion. Unlike supplemental UIP, casein infusion does not appear to cause DM intake to increase. Increased DM intake contributes significantly to the milk production response observed with UIP supplementation. In fact, more than half of the response observed from feeding UIP can be accounted for by the indirect effect of increased energy supply rather than the direct effect of additional AA (20).
Lysine appears to be the first limiting AA when corn or barley based diets are supplemented with cereal based sources of UIP (corn gluten meal, brewers' grains, and distillers grains). Methionine is likely to be the first limiting AA when legume or animal based proteins are the main sources of UIP. In the series of experiments conducted by Schwab and coworkers (28, 29), the need for supplemental lysine was relatively more important than methionine in early and peak lactation (2.5:1 and 2:1, respectively). By midlactation, lysine and methionine tended to be co-limiting. This is supported by the variable response observed when rumen-protected methionine was supplemented alone. Similar studies where lysine is the sole supplemented AA are not available. This is probably due to the greater commercial availability of rumen-protected methionine products compared to lysine.
Because lysine and methionine are considered to be the first limiting AA, virtually all supplementation studies have concentrated on these two AA. Schwab et al. (30) found methionine and lysine supplementation to produce about half of the milk production response of casein or combinations of 10 essential AA for cows in midlactation. In a subsequent study, Schwab et al. (28) found that methionine/lysine supplementation produced 100% of the casein response in early lactation and 47 to 58% of the casein response in peak to midlactation. In virtually every study where infused or rumen-protected methionine and lysine were used, milk protein yield and/or milk protein content increased with supplementation with responses ranging from 4 to 15% (28).More importantly, the increases observed in milk protein tend to be in the casein protein fraction, which has significant importance in cheese production.
Responses to methionine/lysine supplementation in milk yield have been more variable. Some researchers have observed increased milk production with methionine/lysine supplementation (24, 25), whereas others have not (2, 28, 29). Milk fat content is generally not affected by AA supplementation. However, methionine and its hydroxy-analog (MHA) have been used to increase ruminal fiber digestion and alleviate milk fat depression (16). Therefore, methionine that is not fully protected from ruminal degradation may contribute to increased milk fat synthesis. Methionine is also used by the body in fat metabolism and synthesis. Therefore, the response to AA supplementation, in particular, methionine, can be affected by stage of lactation, animal body condition, and diet. This points out that it is often difficult to predict responses from supplementing a nutrient that has many metabolic roles.
A major problem in studying the response to AA supplementation is knowing what the AA requirements of cattle are. In an excellent review, Schwab (27) described three methods of evaluating AA requirements. In addition, AA requirements can be expressed as absolute (grams per day) or relative (proportions or profiles) values. The Cornell Net Carbohydrate and Protein System (CNCPS) is the most dynamic model in use to date. It uses a factorial approach and expresses requirements in both absolute and relative values. Using the direct dose-response method, Schwab (27) expressed requirements in relative terms and concluded that lysine needs to constitute 15% of total absorbed essential amino acids (EAA) and methionine needs to be at least 5.3% of absorbed EAA only when lysine levels reach the 15% level. These values are well above the levels observed in most well balanced diets using conventional feedstuffs (27).
Using the indirect dose-response approach, Socha and Schwab (32) found 1) milk protein content and yield are more closely linked to lysine rather than methionine supplementation; 2) when lysine levels were low (les then 14% of EAA), methionine supplementation decreased content and yield of milk protein, and 3) it is extremely difficult to meet lysine and methionine requirements simultaneously with conventional feedstuffs.
What are RPAA?
Amino acids can be added directly to the diets of monogastric animals to overcome nutritional deficiencies. However, free-form AA are rapidly degraded by rumen bacteria (22) and are of little or no practical benefit in alleviating AA deficiencies for ruminants. Rumen-protected AA must be either modified or protected in some way so that they are not susceptible to rumen degradation. Several methods have been used to develop commercial RPAA products. Ideally, RPAA should be generally recognized as safe (GRAS) to avoid lengthy governmental clearances. Furthermore, a balance must be achieved so that AA protected from ruminal degradation are still available for intestinal absorption. In addition, these compounds should be stable both when pelleted and when incorporated into silage-based total mixed rations (TMR) in which the pH of corn silage can be as low as 3.6.Various analogs of AA have been tested for resistance to ruminal degradation (3). One of the more tested AA derivatives is methionine hydroxy analog (MHA, Novus Intl., Chesterfield, MO). Test results have been variable, with occasional improvements in milk production and milk fat. Patterson and Kung (23) reported that more than 70% of an initial dose of MHA and only 5% of DL-methionine remained after 12 h of in vitro incubation with ruminal microorganisms. Alimet , a liquid form of MHA, was as stable in the rumen as was the solid prill form of MHA.
Fat has been used to make RPAA, but the total proportion of AA has usually been only about 30% by weight. South Dakota researchers conducted several studies with a fatty acid (58%) and methionine (30%) prill. They had variable results in improving milk production. Munneke et al. (19) concluded that, in their series of studies, encapsulation improved methionine status in the animal, but lysine often became the next limiting AA negating large production responses. Currently, Megalac-Plus (Church and Dwight, Inc., Princeton, NJ) is a commercial formulation which contains 13 g of methionine hydroxy analog and 0.45 kg of Megalac (calcium salt of long chain fatty acid formulation). Other sources of fat and AA are also commercially available.
A potential problem with this method is that AA can be over-protected. Complexes that are extremely inert in the rumen can be indigestible in the small intestine as well. Therefore, a tradeoff exists between good ruminal protection and bioavailability.
Polymers that are pH-sensitive have been used to encapsulate methionine and lysine (2, 26). The polymer is stable at ruminal pH, but breaks down when it is exposed to abomasal pH, thus releasing the free AA for absorption in the small intestine. These products will have reduced efficacy when mixed with silage or TMR. Depending on the critical pH of the coating, these products may have limited usefulness in feeding situations where ruminal pH is low (i.e., high-concentrate diets). Examples of such products are Smartamine M (70% methionine) and ML (15% methionine, 50% lysine) which are combinations of AA and poly (2-vinylpyridine-co-styrene) produced by Rhone-Poulenc Animal Nutrition N.A., Atlanta, GA. Additionally, Mepron M85 (Degussa Corporation, Ridgefield Park, NJ) is another protected methionine product which is coated with compounds that are generally recognized as safe (GRAS). Mepron M85 is not a pH-sensitive product, but instead relies upon a polymer that is resistant to ruminal, but not intestinal digestion.
Recently, a novel method of improving the supply of AA to the lower gut was reported on by Ohsumi et al. (21). These researchers isolated a lysine-accumulating Saccharomyces cerevisiae (a yeast) that, depending on substrates, could accumulate from 4 to 15% of their dry weight as lysine. The majority of lysine was in vacuoles that were stable when incubated with rumen fluid, but immediately released when exposed to pepsin. Thus, feeding this organism could increase the amount of lysine for intestinal absorption.
Metal chelates of AA have been used to improve the bioavailability of minerals. Using the same principle, zinc methionine and zinc lysine have been used successfully as RPAA sources (15). The drawback to using zinc AA chelates is the high level of zinc in the diet. Typical levels of AA supplementation can result in zinc levels being 10 to 20 times above normal.
Practical Benefits to RPAA
It is clear that supplementation of specific AA can improve milk and milk protein production when that particular AA is the first limiting nutrient in the diet. In order to utilize this technology in dairy feeding practices, there must be a niche where the benefits of AA supplementation are greater than the cost of providing the RPAA.RPAA as a Substitute for Dietary Protein
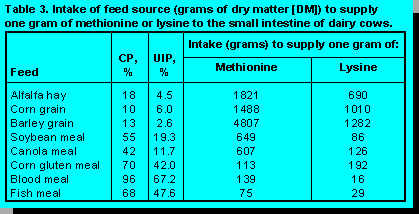 The most obvious role for RPAA is as a substitute for UIP in the diet of dairy cows. Typical diets
for lactating cows can be adequate for UIP and DIP while having a deficiency of 10 to 20 g/day of
lysine and 5 to 10 g/day methionine. One gram of RPAA can provide the same amount of lysine to
the small intestine as 126 g of canola meal (Table 3).
The most obvious role for RPAA is as a substitute for UIP in the diet of dairy cows. Typical diets
for lactating cows can be adequate for UIP and DIP while having a deficiency of 10 to 20 g/day of
lysine and 5 to 10 g/day methionine. One gram of RPAA can provide the same amount of lysine to
the small intestine as 126 g of canola meal (Table 3).
To supply a similar quantity of methionine would require 607 g of canola meal. Alternatively, smaller quantities of blood meal or fish meal could be used to supply the same amount of AA. However, these protein supplements are more costly and often reduce the palatability of the ration. Judicious selection of protein supplements can provide a good balance of methionine and lysine, but adds extra storage and handling requirements for the additional feed ingredients.
Protein supplements contribute more to the diet than essential AA. Amino acids serve as an important source of energy for the cow and of nitrogen for the rumen microbial population. However, these nutritional factors can usually be supplied with less expense by other sources of energy and low quality protein. When proteins are used as a source of energy, the nitrogen component of the protein is converted to urea in the liver. This process requires additional energy that can have a significant negative impact on the cow. For example, a cow consuming the 126 g of dietary canola meal, equivalent to 1 g of RP lysine, will require an additional 0.6 Mcal of metabolizable energy to convert the excess nitrogen into urea. Providing this amount of energy would require an additional 0.2 kg of barley in the diet.
Another issue is the use of animal byproducts in livestock feeds. While the use animal-derived protein supplements is more an ethical than an economic issue in North America, concerns about "mad cow" disease or bovine spongiform encephalopathy (BSE) in Europe has virtually eliminated meat and blood meal from ruminant diets.
Nitrogen pollution of surface and ground water and environmental acidification from livestock production are increasing problems in many areas of the world. Dairy farming accounts for 56% of ammonia emissions in the Netherlands (7). Rumen-protected AA technology is "environmentally friendly" in that it improves the efficiency of protein utilization for dairy cows. Cows are able to produce the same or more milk while being fed lower quality protein feeds.
Recent research from Cornell University (5) have advocated higher levels of UIP in the diets of dry cows because current feeding recommendations under-estimate the protein requirement for the conceptus. This newer system has resulted in greater milk production and improved fertility. However, Julien et al. (14) found an increased incidence of alert downer cow syndrome in cows receiving high protein diets. Use of RPAA may provide an avenue to supply cows with the AA that are required in the dry period without creating the potential for downer cow syndrome (Rode et al. unpublished results). In fact, it may be that supplementation to feed the proper balance of AA in the close-up dry period is equal or even more important than supplementation during lactation.
A deterrent to the use of RPAA is our limited knowledge for balancing diets for AA requirements. Amino acid requirements will vary with stage of lactation and diet. It is unlikely that it will be cost-effective to feed the same amount of RPAA to all cows in a herd or group as "insurance" against AA deficiencies. Newer diet formulation systems such as the CNCPS (12) should provide us with the tools to utilize RPAA technology effectively.
Energy Intake
The greatest limitation to dairy cow productivity is DM intake. By feeding a relatively small quantity of RPAA, it is possible to eliminate a much larger quantity of protein supplement from the diet (Table 3). This makes room in the total diet for other ingredients such as forage or concentrates. Rode et al. (25) replaced 0.5 kg of a soy/blood meal combination with 50 g of RPAA. Both groups of cows had similar DM intake and milk production. Cows supplemented with RPAA consumed less protein and more forage than protein-supplemented cows. Having more room in the diet offers producers much more flexibility in diet formulation.
Fat supplements are fed as an energy-dense substitute for concentrates in an attempt to overcome the limitation on DM intake.A major drawback to feeding supplemental fat is that milk protein content is often reduced. Cant et al. (8) have proposed that this reduction is due to a decreased blood flow to the mammary gland when dietary fat is supplemented. This, in turn, results in fewer plasma AA available for absorption and subsequent protein synthesis. Additionally, dietary fat does not contribute to microbial protein synthesis. Therefore cows are susceptible to a protein and AA deficiency relative to the energy available for milk production. Attempts to overcome the milk protein depression by feeding additional UIP have been unsuccessful (13). Rumen-protected AA supplementation has been successful in overcoming milk protein depression in some studies (9), but not in others (15).
Conclusions
The concept of RPAA supplementation has been with us for a long time. In the near future, several commercial sources of rumen protected lysine and methionine will be available to dairy producers. In numerous studies, AA supplementation has been shown to be effective in improving milk yield and/or milk protein synthesis. Additionally, RPAA provide dairy producers with the opportunity for more flexibility in their feeding systems. The improvement in nitrogen utilization place RPAA as products that fit well in modern environmentally sensitive production systems.Effective use of RPAA technology as well as "bypass" proteins requires knowledge of 1) amount and AA composition of rumen microbial protein, 2) precise AA requirements of the cow, and 3) an effective delivery system for supplemental AA.
Additional Readings
Applied Dairy Science Course - University of Alberta:Energy and Protein Metabolism of Dairy Cattle
Alberta Dairy Management Fact Sheet:
Bypass Protein -
1. Background
References
1. Ainslie, S.J., D.G. Fox, T.C. Perry, D.J. Ketchen and M.C. Barry. 1993. J. Anim. Sci. 71:1312-1319.2. Armentano, L.E., S.M. Swain and G.A. Ducharme. 1993. J. Dairy Sci. 76:2963-2969.
3. Ayoade, J.A., P.J. Buttery and D. Lewis. 1982. J. Sci. Fd. Agric. 33:949-956.
4. Belitz, H.D. and W. Grosch. 1987. Reactions involved in food chemistry. In: Food Chemistry. Springer-Berlug, Berlin, Germany. pp. 53-75.
5. Bell, A.W., M.B. Rymph, R. Slepetis, W.A. House, and R.A. Erhardt. 1992. Proc. Cornell Nutrition Conf. Feed Manuf. Ithaca, NY. pp. 102-109.
6. Benchaar, C., R. Moncoulon, C. Bayouthe and C. Vernay. 1993. J. Anim. Sci. 72: 492-501.
7. Berentsen, P.S., G.W. Geisen and R.F. Speelman. 1993. J. Dairy Sci. 76:2332-2343.
8. Cant, J.P., E.J. DePeters and R.L. Baldwin. 1993. J. Dairy Sci. 76: 2254-2265.
9. Casper, D.P. and D.J. Schingoethe. 1989. J. Dairy Sci. 72: 3327-3335.
10. Clark, J.H., T.H. Klusmeyer and M.R. Cameron. 1993. J. Dairy Sci. 75:2304-2322.
11. Ferguson, J.D., D.K. Beede, R. Shaver, C.E. Polan, J.T. Huber and P.T. Chandler. 1994. J. Anim. Sci. 72(Suppl. 1):238.
12. Fox, D.G., C.J. Sniffen, J.D. O'Connor, J.B. Russell and P.J. Van Soest. 1992. J. Anim. Sci. 70:3578-3596.
13. Hoffmann, P.C., R.R. Grummer, R.D. Shaver, G.A. Broderick and T.R. Drendel. 1991. J. Dairy Sci. 74: 3468-3472.
14. Julien, W.E., H.R. Conrad and H.R. Redman. 1977. J. Dairy Sci. 60:210-215.
15. Kincaid, R.L. and J.D. Cronrath. 1993. J. Dairy Sci. 76: 1601-1606.
16. McCracken, B.A., M.B. Judkins, L.J. Krysl, D.W. Holcomb and K.K. Park. 1993. J. Anim. Sci. 71:1932-1939.
17. Meijer, G.A.L., van der Meulen, J. and van Vuuren, A.M. 1993. Metabolism 42:358-364.
18. Merchen, N.R. and E.C. Titgemeyer. 1992. J. Anim. Sci. 70:3238-3249.
19. Munneke, R.L., D.J. Schingoethe and D.P. Casper. 1991. J. Dairy Sci. 74:227-233.
20. Oldham, J.D. 1984. J. Dairy Sci. 67:1090-1114.
21. Ohsumi, T., H. Sato, Y. Yoshihara and S. Ikeda. 1994. Biosci. Biotechnol. Biochem. 58:1302-1305.
22. Onodera, R. 1993. Amino Acids 5:217-232.
23. Patterson, J.A. and L. Kung Jr. 1988. J. Dairy Sci. 71:3292-3301.
24. Robinson, P.H., A.H. Fredeen, H. Sato, H. Suzuki, W. Chalupa and W.E. Julien. 1993. J. Dairy Sci. (Suppl. 1). 76:203.
25. Rode, L.M., T. Fujieda, H. Sato, H. Suzuki, W. Chalupa and W.E. Julien. 1993. J. Dairy Sci. (Suppl. 1). 76:277.
26. Rulquin, H., L. L. LeHenaff and R. Verite. 1990. Reprod. Nutr. Dev. (Suppl. 2). 30: 238.
27. Schwab, C.G. 1995. 36th Annual Dairy Feed Conference. pp. 1-30.
28. Schwab, C.G., C.K. Bozak, N.L. Whitehouse and M.M.A. Mesbah. 1992. J. Dairy Sci. 75: 3486-3502.
29. Schwab, C.G., C.K. Bozak, N.L. Whitehouse and V.M. Olson. 1992. J. Dairy Sci. 75: 3503-3518.
30. Schwab, C.G., L.D. Satter and A.B. Clay. 1976. J. Dairy Sci. 59: 1254-1265.
31. Smith, S.I. and J.A. Boling. 1984. J. Anim. Sci. 58:187-193.
32. Socha and Schwab. 1995. J. Dairy Sci. 78: (In Press).
33. Stern, M.D., Calsamiglia, S., and Endres, M.I. 1994. Proc. Cornell Nutrition Conf. Pp. 105-116.
34. Storm, E. And Orskov, E.R. 1983. Br. J. Nutr. 52:613-620.
35. Theurer, C.B. J.T. Huber and F.A.P. Santos. 1995. Proc. Southwest Nutrition and Management Conf. pp. 59-67.
36. Waghorn, G.C. and Baldwin, R.L. 1984. J. Dairy Sci. 67:531-538.