Introduction
A comprehensive plan for mastitis control has been widely adopted for reducing the incidence of this disease in dairy cows. The plan includes pre- and postmilking teat dipping, dry cow therapy, treatment of clinical mastitis cases, proper milking hygiene, use of adequately functioning milking equipment, and culling of chronically infected cows. Two of the above points involve the use of antibiotic therapy to eliminate and/or prevent udder infections. Thus, antibiotics have played a historic role in attempts to control the incidence of intramammary infections. Deciding whether to treat clinical mastitis depends on the economic value of the cow in the milking herd, i.e., her age, conformation, past performance, and reproductive status, as well as the costs and benefits of treatments, the value of discarded milk, the probability of treatment failure, likelihood of relapse, the cow's present value as a cull animal, availability of replacement animals, and the risk of antibiotic contamination of milk and meat.The goals of antimicrobial therapy in the treatment of clinical mastitis include the return of the cow to normal milk production and composition, prevention of mortality in peracute cases, elimination of infectious microorganisms, and elimination of practices that may lead to drug residues in milk or meat. The implementation of mastitis control programs, judicious use of antibiotics and other medications, and appropriate record keeping practices will ensure that these goals are met.
Some producers and veterinarians have decided that the risks of antibiotic use in treating clinical mastitis exceed the benefits and have stopped treating clinical cases with antibiotics in herds with a low prevalence of contagious organisms. Instead, they emphasize frequent milkout aided by oxytocin injections and anti-inflammatory drugs, along with emphasis on the management of housing, bedding, and premilking hygiene.
Definitions
At times, there is some confusion in the discussion of clinical mastitis because different meanings are applied to the same term. Subacute clinical mastitis is a condition in which abnormalities of the udder and/or secretion are readily observable. This form of mastitis can vary in severity, depending in part, on the type of microorganism causing the infection. Changes in milk, such as flakes, clots, and watery appearance are the most obvious abnormalities; heat, swelling, and sensitivity of the udder are slight or absent. This is the most common form of clinical mastitis, and is the type on which most treatment efficacy trials are based.Acute mastitis is a condition characterized by sudden onset, redness, swelling, hardness, pain, grossly abnormal milk, and reduced milk yield. Systemic symptoms may also be present and include fever, loss of appetite, reduced rumen function, rapid pulse, dehydration, weakness, and depression. When the onset of disease is very rapid and the signs are very severe, the disease is termed peracute mastitis.
Prevalence of Clinical Mastitis
In a multi-herd study (5), it was demonstrated that herds with mean somatic cell counts (SCC) <150,000/ml had a greater incidence of clinical mastitis than herds with SCC >700,000/ml. In low SCC herds, most clinical cases were caused by environmental streptococci and coliforms. In surveys on well managed herds with SCC <150,000/ml having little mastitis due to contagious pathogens (Staphylococcus aureus and Streptococcus agalactiae), it was shown that 35 to 55% of lactations had one or more incidents of clinical mastitis. In these herds, 15 to 40% of the clinical cases had no bacteria isolated from the milk, 21 to 43% had coliforms, and nine to 32% had environmental streptococci. Overall, prevalence of clinical cases was <4% of quarters (2, 5, 8, 13, 21). In contrast, high SCC herds (>700,000/ml), had a high prevalence of clinical mastitis caused by S. aureus (6.6%) and S. agalactiae (22.2%) (5).Although clinical coliform cases are most prevalent in low SCC herds, only one intramammary antibiotic preparation on the market in the U.S. (hetacillin, not available in Canada) claims efficacy against Escherichia coli (22).
Spontaneous Recovery and Culling
Some cows will eliminate clinical cases of mastitis spontaneously, but others will require culling or drug therapy. The role and limitations of each are discussed. When the cow cures herself of a clinical infection, it is referred to as spontaneous recovery. Research has shown that this occurs in about 20 to 50% of confirmed infections. Most spontaneous recoveries occur in quarters with mild or recent infections and only rarely in the case of well established or chronic infections. It is likely that soon after an infection is established, changes in the cows' immune system take place in an attempt to eliminate the infecting microorganisms. For example, leukocyte numbers as well as their antimicrobial activity become elevated, and with the help of antibodies locally produced in the udder, function to overcome the infection. Spontaneous recovery is enhanced in vaccinated cows, and it is probable that the elevated antibody concentrations in milk are responsible, in part, for such cures.Culling is often the only practical means to eliminate chronic infections from a herd. Research has shown that about 40% of all clinical mastitis was accounted for by just 7% of cows. Other research showed that 50% of all discarded milk was from only 6% of cows. Studies have revealed that 64% of cows that have had two cases of clinical mastitis in the current lactation will have another clinical episode before the end of lactation. The figure increases to 70% for cows that have already had three clinical cases. Moreover, older cows have more clinical mastitis than younger cows. See Philpot and Nickerson (20) for a review. Consideration should be given to culling those repeat offenders that are a constant nuisance and whose continued presence in the herd constitutes a reservoir of microorganisms that may ultimately spread to uninfected cows.
Drug Therapy in General
Spontaneous recovery and culling have serious limitations in terms of usefulness for eliminating infections. This leaves drug therapy as the principal alternative for eliminating existing infections from a herd. By eliminating infections, it is possible to reduce the level of mastitis in months rather than years.During the 1930's and 1940's when sulfonamides and antibiotics were first introduced, there was hope that these drugs would soon put an end to mastitis. Though they were of unquestionable value, the initial optimism faded when it became evident that many infections could not be cured. The ability of microorganisms to develop resistance to drugs was also demonstrated. Drugs do, however, continue to play an important role in mastitis control. Not only are they useful in curing many cows of existing infections, they can save the life of some cows.
The primary concern of most dairy farmers is how to make the best use of antibiotics and other drugs in treating mastitis, or whether to treat at all. Such cases require prompt and appropriate attention, though each case must be considered on an individual cow basis.
Available Drugs and Therapeutic Considerations
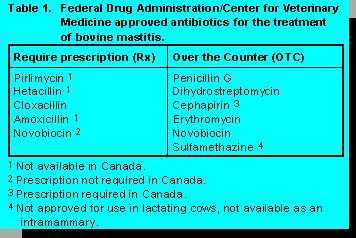 In the U.S., there are currently 16 products marketed for bovine mastitis treatment, six requiring a
prescription. Prescription products include pirlimycin, hetacillin, cloxacillin, amoxicillin, and
novobiocin preparations, and over the counter compounds include penicillin G,
dihydrostreptomycin, cephapirin, erythromycin, novobiocin, and sulfamethazine (nonlactating
dairy cows only) preparations (Table 1).
In the U.S., there are currently 16 products marketed for bovine mastitis treatment, six requiring a
prescription. Prescription products include pirlimycin, hetacillin, cloxacillin, amoxicillin, and
novobiocin preparations, and over the counter compounds include penicillin G,
dihydrostreptomycin, cephapirin, erythromycin, novobiocin, and sulfamethazine (nonlactating
dairy cows only) preparations (Table 1).Producers and veterinarians must be familiar with the regulatory guidelines regarding extra-label use of drugs (ELUD) as they apply to food animals (Table 2). Extra-label use of drugs is permitted under the following conditions:
These guidelines prohibit the off-label use of antibiotics and other drugs by the producer without a valid prescription. Altering the dose of the drug used, the route of administration, and the intended use of the compound is illegal unless there is a valid veterinarian/client/patient relationship. If the compound is used off-label, the veterinarian must recommend an extended withdrawal time to avoid milk or meat residue violations.

General Thoughts on the Treatment of Clinical Mastitis
Animal health and well-being considerations usually dictate that treatment of clinical mastitis be implemented before obtaining culture and sensitivity results. Therefore, selecting an initial treatment regimen for clinical mastitis cases should be based upon historical culture and sensitivity results, severity of infection, and documented success of previous treatments. This approach requires advanced planning, implementation of the treatment program, and accurate treatment records.
Antibiotic therapy as well as other treatment methods are useful in reducing the severity of the clinical condition and perhaps elimination of the infection. Treatment of clinical mastitis alone will not reduce the prevalence of mastitis in the herd unless it is accompanied by all other essential parts of a good mastitis control program.
Mild cases of clinical coliform mastitis may respond well to "stripping out" the infected quarter on a frequent basis, with the use of oxytocin administration to assist in milk let-down. This approach to mastitis therapy may not be a medically sound strategy for environmental streptococci, S. agalactiae, or S. aureus. If abnormal clinical signs persist, or in moderate cases with marked swelling of the gland, commercial intramammary antimicrobial preparations may be indicated. In general, the choice of an antimicrobial agent and the route of administration will be dictated by characteristics of the drug and regulatory issues. Careful monitoring and documentation of both clinical as well as microbiological cure rates are invaluable in maintaining sound medical and economical therapeutic decisions. Antimicrobial therapy is of questionable benefit in recurring or persistent infections, and repeated attempts to treat these infections is not likely to result in therapeutic benefit. Reports suggest that 70% of lost marketable milk resulting from clinical mastitis is due to withholding milk during and after treatment, as opposed to 30% from decreased milk production.
Reasons for Treatment Failures
Mastitis results after bacteria penetrate teat duct keratin, overcome the defenses in milk, and multiply within the gland. The interaction of bacteria with milk leukocytes greatly influences the establishment of infections. These leukocytes function by phagocytosing bacteria and killing the organisms intracellularly. However, phagocytosis is inefficient in the udder due to the lack of an energy source, low opsonic activity, and interference caused by casein and butterfat. Thus, antibiotics continue to be relied upon in attempts to treat clinical quarters during lactation. Reasons for treatment failure include lack of contact between bacteria and antibiotics due to scar tissue formation, protection within leukocytes, poor drug diffusion, and inactivation by milk and tissue proteins; microbial resistance to antibiotics; development of bacterial L-forms; metabolically inactive organisms; and improper treatment procedures, i.e., stopping therapy too soon.For effective intramammary therapy, it is necessary for antibiotics to reach sites of infection at concentrations exceeding the minimum inhibitory concentration (MIC) of the drug and remain at adequate concentrations for sufficient time to kill or inhibit growth of the infective agent. Unfortunately, therapeutic concentrations may not be achieved in mammary tissue for a sufficient period of time when presently used doses of antibiotics are administered intramammarily. In one study at the Hill Farm Research Station, using intramammary drugs at the recommended dosage of 3 infusions at 12-hour intervals, cure rates for subclinical infections were 44% against S. aureus and 72% against S. agalactiae. If infusions were administered 6 times at 12-hour intervals, cure rates increased to 50% and 78%.
Infections are frequently refractory to intramammary therapy because of the inaccessibility of bacteria due to deep tissue lesions, swelling, and reduced patency of milk ducts. Studies have shown that distribution of infused antibiotics is poor in mastitic quarters because of the above host responses to infection. The frequent therapy failures during acute mastitis are due, in part, to poor or uneven distribution of the drug throughout the intensely swollen udder parenchyma in which the duct system is either compressed or blocked by inflammatory products.
One of the hallmarks of acute inflammation is the formation of inflammatory exudates composed of necrotic tissue debris, leukocytes, bacteria, fibrin, and other blood components that occlude milk ducts draining areas of secretory tissue. If bacteria and their toxins are eliminated by host defenses combined with antibiotic therapy, inflammation subsides, occluded ducts open, and milk composition returns to normal in several days or weeks. However, if occluded areas are not flushed at every milking and continued bacterial growth and toxin production occur, leading to more severe injury, bacteria continue to multiply and become inaccessible to drug action. For example, during chronic S. aureus mastitis with clinical flare-ups, abscesses originating in occluded areas of small mammary ducts are often formed that wall-off the bacteria. This walling-off phenomenon may be a normal defense mechanism to keep the infecting organism contained, but is partially responsible for poor cure rates.
Therapy of Subacute Clinical Mastitis
Most cases of clinical mastitis fall into this category. The intensity of treatment is reduced in comparison to acute toxic mastitis. In fact, intramammary infusion with an approved product for a minimum of three days, accompanied by frequent hand stripping to remove secretion and debris, is often adequate. Treatments should be continued until at least 24 hours after the disappearance of clinical symptoms. Otherwise, the infection may only be suppressed back to the subclinical level. A true cure, whereby all infecting microorganisms are eliminated from the affected quarter, occurs in only 10 to 50% of cases. The cure rate is dependent on how long the infection has been present, age of the cow, type of organism involved, and other factors.
Antibiotic Therapy of Specific Mastitis Pathogens
Streptococcus agalactiae is highly sensitive to, and easily cured by, approved intramammary antibiotics used according to the label. Drug use is easily justified because it stops the shedding of bacteria by the cow with clinical mastitis and because S. agalactiae is very sensitive to all of the antibiotic preparations on the market for lactating cows.While all mastitis infusion products carry a label claim for S. aureus, the cure rate is so low that dairymen are best advised to consider it negligible when following label instructions. The best hope for successful antibiotic treatment is in young cows with recent infections and in heifers before they freshen. However, research results on the use of combination therapy and/or extended therapy has also been promising. In herds with a high prevalence of S. aureus infections, the emphasis should be placed on teat dipping, culling, milking machine maintenance, milking hygiene, and segregation of infected cows to gradually reduce prevalence of infection. Antibiotic treatment may reduce shedding of bacteria by clinical cows, and thus help reduce the spread, but it will not reduce overall prevalence in the herd (19).
In herds with low SCC and low prevalence of contagious pathogens, about 15 to 40% of pretreatment milk samples from cows with clinical mastitis are negative for bacterial growth (1, 2, 5, 8). These samples containing too few organisms for a positive culture may reflect the ability of the cow's immune system to rid the affected quarter of pathogens. The aim of treatment should be to return the quarter and the milk to normal, thus, anti-inflammatory drugs or immune modulators would seem indicated, rather than antibiotics.
In other surveys of clinical mastitis in herds with low SCC, coliform organisms account for about one-third of the isolates from clinical cows. Efficacy studies (4, 7) have shown that treatment of these cows with intramammary gentamicin (not approved for lactating cows) did not affect clinical outcomes. Most of the clinical signs of coliform mastitis are thought to be due to the effects of endotoxin. Treatment should therefore aim primarily at removing endotoxin from the udder with frequent and complete milkout using oxytocin, and at counteracting the effects of endotoxin with appropriate anti-inflammatory and supportive treatments, such as fluids and calcium (6).
Environmental streptococci and coliforms account for the majority of "environmental" clinical mastitis. Philpot (19) reported a cure rate of 36% after intramammary treatment for mastitis caused by environmental streptococci, while Erskine (4) found that acceptable cure rates of >75% are attainable with a combination of intramammary antibiotics and intramuscular procaine penicillin G. Tyler (24) contends that response of clinical Streptococcus uberis infections to antibiotic therapy is poor, although a combination of parenteral and intramammary erythromycin appears promising.
Therapy of Acute Toxic Mastitis
Acute toxic mastitis is most frequently caused by coliform bacteria, though other microorganisms may also cause severe cases. When such cases occur, the dairy veterinarian should be called immediately. Successful therapy must be directed primarily against the effects of endotoxins, which result in severe depression, progressive dehydration, inability to stand, and diarrhea. Treatment procedures recommended are summarized as follows:
In selecting antibiotics or sulfonamides for use in treating such cases, the veterinarian will base selection on previous experience in the herd and on clinical signs and environmental circumstances. The selection is made easier if milk samples are collected from all clinical cases before initiating therapy for culture in a laboratory to identify the microorganisms. Drug resistance testing can also be conducted on such microorganisms to guide in making decisions on therapy of future cases.
Nursing Care and Supportive Therapy
There is no substitute for good nursing care in hastening recovery from clinical mastitis. The provision of fresh drinking water, high quality hay, and a clean, dry, well ventilated environment are important. Also, the frequent hand stripping of affected quarters aids in removing toxic substances resulting from infection. Use of the milk let-down hormone, oxytocin, facilitates complete removal of milk, debris, and toxins. In instances in which gangrene develops, surgical removal of the teat by a veterinarian may facilitate drainage of toxic materials and increase the chances of salvaging the cow.Daily production, appearance of milk, and water and feed intake should be monitored and recorded to assess the cow's progress. The more severe clinical cases will experience additional undesirable stress, if for example, temperature extremes exist in the hospital environment.
The cow exhibiting clinical shock requires additional symptomatic support beyond antimicrobial therapy. Various regimens of normal electrolyte or fluid therapy administered via intravenous drip, corticosteroid therapy, and nonsteroidal anti-inflammatory compounds administered intravenously have been used under veterinary care. If corticosteroids are used, the chances of a subsequent bacteremia are increased; therefore, extreme caution in the use of these compounds should be exercised. Some cows may become hypocalcemic during the course of the disease. Careful administration of calcium solutions may be undertaken if serum biochemistry values warrant such therapeutic measures. These cows are very susceptible to cardiac arrhythmias, cardiac failure, and death if calcium is administered too rapidly or when it is not indicated.
Combination Therapy
The general lack of therapeutic success against clinical mastitis has prompted a reevaluation of treatment strategies. Despite the availability of several antibiotics with good in vitro activity, cure rates are poor, suggesting that inadequate concentrations of active antibiotic are coming into contact with the infecting bacteria for sufficient time to be effective. Antibiotic concentrations exceeding the MIC in milk have been accepted to be effective therapeutic concentrations. Mastitis is, however, a disease of the mammary tissue. This is especially pronounced in the case of S. aureus, which is considered a deep tissue invader, and antibiotic concentrations attained in milk will have only weak, if any, correlation to the actual concentrations present in the foci of S. aureus-infected tissues. Thus, the most effective therapeutic method for treating intramammary infections may be via systemic administration of antibiotic combined with intramammary infusion.Determination of antibiotic concentrations in mammary tissue biopsies from cows receiving various treatments at the Hill Farm Research Station indicated that intramammary infusion alone did not achieve adequate antibiotic concentrations in deep parenchymal tissue for a sufficient period of time to eliminate established infections. Gross histologic evaluation of dye-infused, chronically infected glands revealed poor penetration of the dye throughout the parenchyma, suggesting a similar fate for the infused antibiotic. Combination of multiple intramuscular injections with intramammary infusions over a three-day period resulted in the highest tissue antibiotic concentrations.
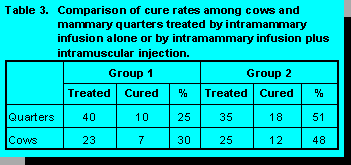 A subsequent study demonstrated that combination therapy was more effective in curing chronic
S. aureus infections with clinical flare-ups than intramammary infusion alone during lactation
(18). In this study, 49 cows with 78 infected quarters were allotted to two treatment groups. One
group of cows received intramammary infusion at each milking for six milkings with a lactating
cow product containing 62.5 mg amoxicillin. Another group of cows received the same
intramammary infusion regimen plus one intramuscular injection daily of nine million units of
procaine penicillin G for three days. The combination of intramammary and intramuscular
treatment cured 51% of quarters (48% of cows) compared with 25% of quarters (30% of cows) for
intramammary infusion alone (Table 3). Thus, combination therapy was twice as effective as
conventional infusion alone, and allowed more antibiotic to penetrate deep areas of infection,
increasing the cure rate. Somatic cell counts taken prior to initiation of therapy from milk of cured
quarters averaged 2,501,000 and were lower than those from failed quarters (4,047,000). By
eight days after treatment, SCC for cured quarters was 340,000 compared with 1,900,000 for
failed quarters. At 21 days after treatment, SCC were 224,000 for cured quarters and 1,975 x
103 for failed quarters.
A subsequent study demonstrated that combination therapy was more effective in curing chronic
S. aureus infections with clinical flare-ups than intramammary infusion alone during lactation
(18). In this study, 49 cows with 78 infected quarters were allotted to two treatment groups. One
group of cows received intramammary infusion at each milking for six milkings with a lactating
cow product containing 62.5 mg amoxicillin. Another group of cows received the same
intramammary infusion regimen plus one intramuscular injection daily of nine million units of
procaine penicillin G for three days. The combination of intramammary and intramuscular
treatment cured 51% of quarters (48% of cows) compared with 25% of quarters (30% of cows) for
intramammary infusion alone (Table 3). Thus, combination therapy was twice as effective as
conventional infusion alone, and allowed more antibiotic to penetrate deep areas of infection,
increasing the cure rate. Somatic cell counts taken prior to initiation of therapy from milk of cured
quarters averaged 2,501,000 and were lower than those from failed quarters (4,047,000). By
eight days after treatment, SCC for cured quarters was 340,000 compared with 1,900,000 for
failed quarters. At 21 days after treatment, SCC were 224,000 for cured quarters and 1,975 x
103 for failed quarters.
Further studies with ceftiofur, a third generation cephalosporin currently available for treatment of bovine respiratory disease, showed that intramuscular injections of 500 mg resulted in milk and mammary tissue concentrations below detectable limits. Staphylococcus aureus was not eliminated from infected mammary glands by infusion of 100 mg ceftiofur or injection of 500 mg ceftiofur by 48 h after treatment. Combination therapy of 100 mg ceftiofur infused and 500 mg injected reduced S. aureus numbers in milk and mammary tissue markedly, as did infusion of 200 mg ceftiofur. Cows receiving intramammary infusion of 200 mg ceftiofur (two doses at 24-hour intervals) had highest concentrations in milk and in tissue. These concentrations were similar to those obtained with two 200 mg doses of cephapirin at 24-hour intervals. Results of repeated treatments and combination therapy suggested that quarters exposed to higher concentrations of antibiotic regained secretory activity at a greater rate than those treated by infusion or injection alone.
In a review by Kirk (15), combining quarter infusions with systemic treatment increased the clinical cure rate from 20 to 50% in the lactating cow. Suggestions for combination treatments included: 1) ampicillin at 10 mg/kg twice daily given intramuscularly and continued for 3 to 5 days; 2) erythromycin at 5 mg/kg given intramuscularly twice daily, or 300 mg twice daily administered intramammarily and continued for 3 to 5 days; and 3) oxytetracycline at 20 mg/kg daily given either intravenously or intramuscularly.
Field Studies on the Efficacy of Therapeutic Regimens
In studies dealing with cure rate for clinical mastitis, the majority of which is subacute, investigators express efficacy as either bacteriological cures (the absence of the infecting organism after a certain time point after treatment) or clinical cures (the return of the affected quarters to visibly normal milk, with or without the presence of the infecting organism).Results from three multi-location commercial field trials on the therapy of clinical mastitis were recently reported (12). Each trial compared the use of investigational drugs with either no treatment or with a positive control, and included approximately 40 herds and 1,400 cows. In these studies, clinical mastitis was defined as visibly abnormal milk with or without additional clinical symptoms. Milk from treated cows was observed at each milking and defined as normal or abnormal based on visual observation through 23 milkings after initial treatment. In addition, bacteriological analysis and SCC were determined throughout the trial. A quarter was defined as cured if milk was visibly normal by the 23rd milking after treatment, no udder abnormalities were apparent, and the absence of mastitis pathogens upon microbiologic culture.
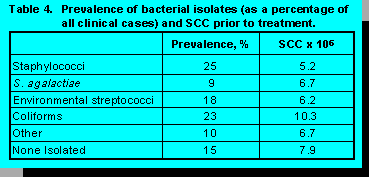 The prevalence of bacterial isolates from the clinical cases observed prior to treatment is shown in
Table 4. Positive microbiological cultures were present in 79% of the samples. Based on these
positive samples, over 50% of infections were caused by the staphylococci and streptococci; in
15% of samples, no organism could be isolated. Somatic cell counts ranged from 5.2 to 10.3
million cells/ml.
The prevalence of bacterial isolates from the clinical cases observed prior to treatment is shown in
Table 4. Positive microbiological cultures were present in 79% of the samples. Based on these
positive samples, over 50% of infections were caused by the staphylococci and streptococci; in
15% of samples, no organism could be isolated. Somatic cell counts ranged from 5.2 to 10.3
million cells/ml.
The number of quarters from which no pathogens could be isolated at the 23rd milking relative to the total number enrolled in the trial was also determined. The number of apparently cured quarters (166/291) was greater in quarters receiving antibiotic treatment (57%) than in nontreated controls (97/272, 37%). Treatment resulted in a 20% numerical improvement in the percentage of quarters without mastitis pathogens. If these numbers are presented as an improvement using control values as baseline, then antibiotic therapy provided a 54% increase in the number of negative quarters by the 23rd milking.
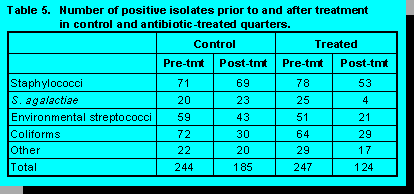
After treatment, the number of mastitis-causing bacteria isolated from previously clinical quarters was reduced as shown in Table 5.
 Data also indicated that antibiotic treatment increased the percentage of cows returning to normal
milk by the 23rd milking (64 versus 77%). Regardless of treatment status, cows returned to
visually normal milk in eight to 11 milkings. The presence or absence of mastitis pathogens did
not alter the time for return-to-normal milk. A higher percentage of antibiotic-treated cows with
normal milk (68%) had no mastitis pathogens present in their milk compared with nontreated
control cows (43%). In addition, quarter cure rates, assessed by time to return-to-normal milk,
absence of udder clinical signs, and lack of mastitis pathogens showed that cures were greater for
the treated quarters compared with controls (51 versus 28%).
Data also indicated that antibiotic treatment increased the percentage of cows returning to normal
milk by the 23rd milking (64 versus 77%). Regardless of treatment status, cows returned to
visually normal milk in eight to 11 milkings. The presence or absence of mastitis pathogens did
not alter the time for return-to-normal milk. A higher percentage of antibiotic-treated cows with
normal milk (68%) had no mastitis pathogens present in their milk compared with nontreated
control cows (43%). In addition, quarter cure rates, assessed by time to return-to-normal milk,
absence of udder clinical signs, and lack of mastitis pathogens showed that cures were greater for
the treated quarters compared with controls (51 versus 28%).
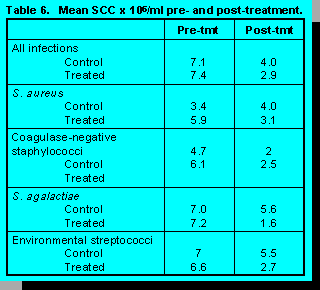
Table 6 presents pre- and post-treatment SCC data for all infected quarters and for specific pathogens. Treatment provided a greater reduction in SCC post-treatment compared with nontreated control cows. For nontreated control cows, SCC increased for cows infected with S. aureus and S. agalactiae from pre- to post-treatment time points.
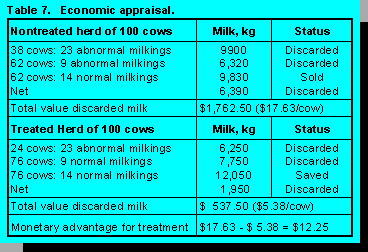
The economics of treatment was determined utilizing the data presented above and several assumptions, which included the following:
Using the 23-milking post-treatment interval, a cow without mastitis would produce 260 kg (23 milkings x 11.3 kg) of milk during this interval worth $71.88. If two theoretical 100-cow herds are created (Table 7), and one is a nontreated herd and the other is an antibiotic-treated herd, the treated herd would discard 4,439 kg less milk. Over the 23-milking period, this is a financial benefit of $1,225 for the entire treated herd ($12.25/cow) over the nontreated control herd.
These data demonstrated that antibiotic therapy of clinical mastitis in the lactating dairy cow reduces the number of pathogens in milk, increases the number of quarters returning to normal milk and the number of cured quarters compared with nontreated controls, and returns a financial benefit of $12.25/cow.
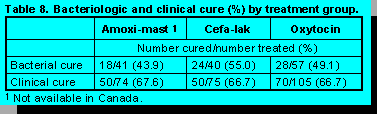 In another three-herd study (11), 254 quarters with mild clinical mastitis were randomly assigned
to three groups. Group 1 (n = 74) was treated with 62.5 mg of intramammary amoxicillin every 12
hours for 3 milkings, Group 2 (n = 75) was treated with 200 mg of intramammary cephapirin
every 12 hours for 2 milkings, and Group 3 (n = 105) was treated with 100 units of intramuscular
oxytocin every 12 hours for 2 or 3 milkings. Microbiologic analysis of pretreatment quarter
samples revealed a frequency of 37% coliforms, 26% environmental streptococci, 13% other
bacteria, and 24% with no isolate. Clinical cure (return of quarter and milk to normal) and
bacterial cure (absence of a primary pathogen isolated pretreatment) were assessed at milking
eight and on day 20 after initial treatment. No difference existed in clinical (67.6, 66.7, 66.7%) or
bacteriologic (43.9, 55.0, 49.1%) (Table 8) cure rate among the 3 treatment groups. However,
clinical cure rates for quarters infected with other pathogens (coagulase-negative staphylococci,
Bacillus spp., Actinomyces spp., and mixed infections) were significantly lower in cows treated
with oxytocin than antibiotic-treated cows.
In another three-herd study (11), 254 quarters with mild clinical mastitis were randomly assigned
to three groups. Group 1 (n = 74) was treated with 62.5 mg of intramammary amoxicillin every 12
hours for 3 milkings, Group 2 (n = 75) was treated with 200 mg of intramammary cephapirin
every 12 hours for 2 milkings, and Group 3 (n = 105) was treated with 100 units of intramuscular
oxytocin every 12 hours for 2 or 3 milkings. Microbiologic analysis of pretreatment quarter
samples revealed a frequency of 37% coliforms, 26% environmental streptococci, 13% other
bacteria, and 24% with no isolate. Clinical cure (return of quarter and milk to normal) and
bacterial cure (absence of a primary pathogen isolated pretreatment) were assessed at milking
eight and on day 20 after initial treatment. No difference existed in clinical (67.6, 66.7, 66.7%) or
bacteriologic (43.9, 55.0, 49.1%) (Table 8) cure rate among the 3 treatment groups. However,
clinical cure rates for quarters infected with other pathogens (coagulase-negative staphylococci,
Bacillus spp., Actinomyces spp., and mixed infections) were significantly lower in cows treated
with oxytocin than antibiotic-treated cows.
Hogan et al. (14) evaluated the efficacy of two antibiotic infusion products for mild clinical cases over an eight-year period. Treatment 1 was a product containing 100,000 units of penicillin and 150 mg of novobiocin infused twice. Treatment 2 was the same product as Treatment 1, but infused three times. Treatment 3 was 200 mg of cephapirin infused twice, and cows in Treatment 4 were untreated controls. Cure rates are shown in Table 9.
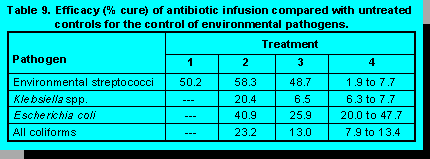 In this study, efficacy was good for the environmental streptococci across all infusion products
used. For those quarters infected with Klebsiella spp., treatment efficacy was low, but therapy
with three infusions of penicillin/novobiocin was more effective than other treatments. Of the two
infusion treatments in the therapy of E. coli mastitis, three infusions of penicillin/novobiocin were
superior to two infusions of cephapirin, but efficacy was within the range of untreated
controls.
In this study, efficacy was good for the environmental streptococci across all infusion products
used. For those quarters infected with Klebsiella spp., treatment efficacy was low, but therapy
with three infusions of penicillin/novobiocin was more effective than other treatments. Of the two
infusion treatments in the therapy of E. coli mastitis, three infusions of penicillin/novobiocin were
superior to two infusions of cephapirin, but efficacy was within the range of untreated
controls.
In another herd study (3), an 87% clinical cure of S. aureus and S. uberis infections were reported in cows receiving no therapy for mild clinical mastitis (cases showing flakes in milk with or without mild swelling of the quarter). However, the bacteriologic cure for both organisms was only 20%.
In one study, treatment of clinical mastitis of unspecified etiology by increasing the milking frequency was compared with treatment with systemic antibiotics (penicillin-streptomycin (not available in Canada), chloramphenicol, or oxytetracycline) (16). The frequent milkout group was milked every 2 to 3 hours daily with an 8-hour rest period at night and had a clinical cure rate of 81%, but a recurrence rate of 53%. The group treated with antibiotics had rates of 85 and 42%, respectively. Thus, no difference was observed in clinical cure or recurrence rates between these treatments. An evaluation of intramammary cephapirin against clinical mastitis cases showed a bacterial cure rate of 21.6% (23). In this herd, 30% of samples showed no growth, 2 of 37 samples from clinical quarters had S. aureus, and the rest had environmental pathogens; no untreated controls were used.
Results of Extended Therapy Regimens
Other field trials, in which the treatment efficacy of both subclinical and clinical cases were evaluated, generally indicate that extended treatments beyond that originally recommended by the manufacturer improved cure rates. In one study (17), on cows having chronic S. aureus mastitis with clinical flare-ups, pirlimycin (not available in Canada) infusions were made at 24-hour intervals followed by a 36-hour withholding time as per the manufacturer's directions. At 48 hours after the last treatment, the treatment regimen was repeated followed by a second 36-hour withholding period. At 48 hours after the last treatment, the regimen was repeated a third time for a total of six infusions per quarter over an 8-day period (15 milkings).
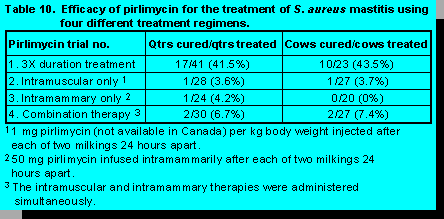 Results of the 3X duration treatment (Table 10) demonstrated that 17 of 41 treated quarters
(41.5%) were cured by day 28 after treatment or 43.5% of cows (Trial 1). This efficacy is much
greater than that observed for pirlimycin used in previous trials 2 through 4 as intramuscular
therapy only, intramammary therapy only, or as combination therapy. Results indicated that this
extended duration therapy regimen increased cure rates against S. aureus infections without
requiring higher daily doses of pirlimycin.
Results of the 3X duration treatment (Table 10) demonstrated that 17 of 41 treated quarters
(41.5%) were cured by day 28 after treatment or 43.5% of cows (Trial 1). This efficacy is much
greater than that observed for pirlimycin used in previous trials 2 through 4 as intramuscular
therapy only, intramammary therapy only, or as combination therapy. Results indicated that this
extended duration therapy regimen increased cure rates against S. aureus infections without
requiring higher daily doses of pirlimycin.
In a study sponsored by the developer of pirlimycin using the extended therapy regimen, a four-herd natural exposure study resulted in an average cure rate for S. aureus cases of 62.9%. Mean SCC were reduced from 3.4 million pretreatment to 280,000/ml in cured cows 4 weeks later.
Other studies at the Hill Farm Research Station have indicated a slight therapeutic benefit of six consecutive infusions (6X) versus three consecutive infusions (3X) given after milking 12 hours apart. Data on five lactating cow products used 3X yielded average cure rates of 44 and 72% for subclinical infections caused by S. aureus and S. agalactiae, and when used 6X, the cure rates were 50 and 78%. Average cure rates for 6X treatment against S. aureus and S. agalactiae clinical mastitis were 29 and 88%.
Intramammary Treatment Procedures
In the local treatment of clinical mastitis, teats of individual quarters that are selected to receive treatment must be clean and dry. Each teat end should be scrubbed thoroughly until clean with cotton soaked in 70% alcohol. If more than one quarter is to be infused, teats on the far side of the mammary gland should be disinfected first, then treated last, after the teats on the near side have been disinfected and infused. During infusion, the cannula should not be inserted completely through the teat duct because this may damage teat duct keratin. The cannula tip should be inserted approximately 1/8 inch into the duct while the therapeutic agent is being administered, and teats should be dipped in a germicidal teat dip after infusion. Single use of syringes and cannulas is essential. If special care is not taken in cleaning and sanitizing teats prior to infusion, microorganisms present on the end of the teat may be forced into the mammary gland, resulting in a more severe infection than the one for which treatment was intended.
Somatic Cell Response to Treatment
The time required for SCC to decrease substantially after successful therapeutic intervention depends upon the type of microorganism involved and the tissue damage resulting from the infection. The range of time required for SCC to return to normal may be a few days for some streptococcal infections, to a few months or even to the next lactation for some infections due to staphylococci. Some quarters may be permanently damaged and can produce milk with elevated SCC for an indefinite period of time.
Antibiotic Resistance Testing
If cure rates appear particularly low when treating clinical mastitis, it is possible that the infecting microorganism is resistant to the antibiotic being infused. For example, S. aureus is often resistant to penicillin, and other antibiotics need to be considered. In such instances, it is advisable to collect milk samples from infected quarters or the bulk milk tank for culture. The diagnostic laboratory or herd veterinarian will then isolate specific microorganisms from the samples and test them against a spectrum of antibiotics or other drugs to determine those to which microorganisms are susceptible or resistant. It is important to remember that microorganisms susceptible to an antibiotic in the laboratory may resist the drug in the udder for the following reasons:
Microorganisms appearing resistant in the laboratory can be assumed refractory to treatment with that antibiotic.
Avoiding Drug Residues
The consuming public has expressed strong disapproval of the presence of illegal drug residues in milk or in meat from cull dairy cows. Because milk is universally produced and widely used in a variety of food products, it is targeted for special monitoring. Every effort must be made by every dairy farmer to insure that milk products and meat are free of all drug residues. There is good reason for concern because some humans are very sensitive to certain drugs. Moreover, it is feared that the presence of drugs in food will result in resistance to drug therapy in humans.Most dairy farmers are very conscientious about taking all possible precautions to avoid drug residues, especially from cows treated for mastitis. To avoid detectable residues in milk, it is imperative that label instructions are followed exactly. Special attention should be given to dose levels, routes of administration, and withdrawal time. In instances in which the veterinarian administers treatment, it is important to obtain written information on the drug used and the withdrawal time.
Written records should also be kept on all treated cows. This information can help in making culling decisions for problem cows with chronic mastitis. Furthermore, it is desirable to have one person designated for handling antibiotics and for treating cows. It is also important that treated animals not be sold for slaughter until the drug withdrawal time for meat has elapsed.
Other Nonantibiotic Approaches to Treatment
Use of hypertonic saline has been used to alleviate clinical symptoms. A 7.5% saline solution is used. Practitioners recommend that 2 mL per 45 kg of body weight be injected intravenously. In addition, 500 to 1000 mL can be infused intramammarily. This is performed once after each milking for two to three days. A 50 to 60% clearing of clinical symptoms is observed in some cases; however, it is likely that the spontaneous cure rate is very similar.Greene et al. (9) studied the effects of treating clinical mastitis with intramammary infusions of either a Lactobacillus (probiotic) or an antibiotic (cephapirin) preparation. The majority of pathogens isolated were Gram-negative bacilli and environmental streptococci. Treatment of quarters with Lactobacillus cured 21.7% of infected quarters, while 73.7% of infections treated with antibiotic were eliminated. Mean SCC increased for Lactobacillus-treated quarters after infusion, while SCC of antibiotic-treated quarters did not change. The results indicate that this Lactobacillus product was not effective as an intramammary treatment for clinical mastitis.
Designing a Treatment Strategy
Protocols for mastitis treatment on dairy farms were recently outlined (10). For example, on farms in which clinical mastitis is caused by S. agalactiae, antibiotic infusion products should be used on all clinical cases. On farms in which one-third of the clinical samples show no growth and one-half yield E. coli, antibiotic use may be justified for only a very few cows. In herds with a high incidence of environmental Gram-positive infections, some combination of intramammary and systemic antibiotics may be effective.It is recommended that dairy personnel should be trained to examine the cow's record before beginning a course of treatment for clinical mastitis (10). A cow that is below the herd average, open, and in late lactation will most likely be culled. An average first lactation cow late in gestation should be dried off early because dry cow preparations are more efficacious than lactating cow products and present less risk of contaminating the bulk tank with antibiotics. Cows with persistent or recurring clinical infections despite past treatment attempts are unlikely to respond to the same treatment protocol. However, a young, high-yielding cow in early lactation with mild mastitis might be treated aggressively, with an emphasis on frequent and complete milkout.
Additional Readings
WCDS-1995:Udder Health is a Management Decision
Applied Dairy Science Course - University of Alberta:
Mastitis in Dairy
Cattle
Alberta Dairy Management Fact Sheet:
Staph Aureus and
Bulk Tank Culturing
References
1. Anderson, K.L., A.R. Smith, B.K. Gustafsson, S.L. Spahr and H.L. Whitmore. 1982. Diagnosis and treatment of acute mastitis in a large dairy herd. J. Am. Vet. Med. Assn. 181-185:690.2. Bennett, R.H. 1990. Clinical mastitis from environmental pathogens: analysis of a large commercial dairy. Pages 181-185 in Proc. Int. Symp. Bov. Mastitis, Am. Assoc. Bovine Pract., Indianapolis, IN.
3. Chamings, R.J. 1984. The effect of not treating mild cases of clinical mastitis in a dairy herd. Vet. Rec. 115:499-505.
4. Erskine, R.J. 1991. Therapy of clinical mastitis: successes and failures. In: Proc. Natl. Mastitis Counc., Inc., Arlington, VA., pp. 30-40.
5. Erskine, R.J., R.J. Eberhart, L.J. Hutchinson, S.B. Spencer and M.A. Campbell. 1988. Incidence and types of clinical mastitis in dairy herds with high and low somatic cell counts. J. Am. Vet. Med. Assn. 192:761-766.
6. Erskine, R.J., J.H. Kirk, J.W. Tyler and F.J. DeGraves. 1993. Advances in the therapy for mastitis. Vet. Clin. North Am. Food Anim. Pract. 9:499-517.
7. Erskine, R.J., R.C. Wilson and M.G. Riddell, Jr. 1990. The pharmacokinetics and efficacy of intramammary gentamicin for the treatment of coliform mastitis. Pages 256-260 in Proc. Int. Symp. Bov. Mastitis, Am. Assoc. Bovine Pract., Indianapolis, IN.
8. Gonzalez, R.N., D.E. Jasper, N.C. Kronlund, T.B. Farver, J.S. Cullor, R.B. Bushnell and J.D. Dellinger. 1990. Clinical mastitis in two California dairy herds participating in contagious mastitis control programs. J. Dairy Sci. 73:648-660.
9. Greene, W.A., A.M. Gano, K.L. Smith, J.S. Hogan and D.A. Todhunter. 1991. Comparison of probiotic and antibiotic intramammary therapy of cattle with elevated somatic cell counts. J. Dairy Sci. 74:2976-2981.
10. Guterbock, W.M. 1994. Rational treatment of clinical mastitis. In: Proc. Natl. Mastitis Counc., Inc., Arlington, VA., pp. 40-50.
11. Guterbock, W.M., A.L. Van Eenennaam, R.J. Anderson, I.A. Gardner, J.S. Cullor and C.A. Holmberg. 1993. Efficacy of intramammary antibiotic therapy for treatment of clinical mastitis caused by environmental pathogens. J. Dairy Sci. 76:3437-3444.
12. Hallberg, J.W., C.L. Henke and C.C. Miller. 1994. Intramammary antibiotic therapy: to treat or not to treat? Effects of antibiotic therapy on clinical mastitis. Pages 28-39 in Proc. Natl. Mastitis Counc., Inc. Arlington, VA.
13. Hogan, J.S., K.L. Smith, K.H. Hoblet, P.S. Schoenberger, D.A. Todhunter, W.D. Hueston, D.E. Pritchard, G.L. Bowman, L.E. Heider, B.L. Brockett and H.R. Conrad. 1989. Field survey of clinical mastitis in low somatic cell count herds. J. Dairy Sci. 72:1547-1556.
14. Hogan, J.S., K.L. Smith, D.A. Todhunter, and P.S. Schoenberger. 1987. Efficacy of antibiotic infusion products for lactational therapy of mastitis caused by environmental pathogens. In: Proc. Int. Mastitis Symposium, MacDonald College, Ste. Anne. PQ, Canada. pp. 1-7.
15. Kirk, J.H. 1991. Diagnosis and treatment of difficult mastitis cases. Agri-Practice. 12:5-8.
16. Opletal, A., D. Rysanek and L. Sladky. 1985. Therapeutic effectiveness of frequent milking out and antibiotic treatment in acute catarrhal mastitis. Pages 414-420 in Proc. V Int. Symp. Mastitis Control, Bydgoszcz, Poland.
17. Owens, W.E., C.H. Ray, R.L. Boddie, N.T. Boddie, C.A. Pratt, S.C. Nickerson, J.W. Hallberg and A.P. Belchner. 1995. Efficacy of sequential pirlimycin intramammary treatment against chronic Staphylococcus aureus intramammary infection. Pages 144-145 in Proc. Natl. Mastitis Counc., Inc. Arlington, VA.
18. Owens, W.E., J.L. Watts, R.L. Boddie and S.C. Nickerson. 1988. Antibiotic treatment of mastitis: comparison of intramammary and intramammary plus intramuscular therapies. J. Dairy Sci. 71:3143-3447.
19. Philpot, W.N. 1979. Control of mastitis by hygiene and therapy. J. Dairy Sci. 62:168-176.
20. Philpot, W.N. and S.C. Nickerson. 1992. Mastitis: Counter Attack. Babson Bros. Co., Naperville, IL. 150 pp.
21. Schukken, Y.H., F.J. Grommers, D. van de Geer and A. Brand. 1989. Incidence of clinical mastitis on farms with low somatic cell counts in bulk milk. Vet. Rec. 125:60-68.
22. Sundlof, S.F., J.E. Riviere and A.L. Craigmill. 1991. Food Animal Residue Avoidance Databank Trade Name File. Inst. Food Agric. Sci., Univ. Florida, Gainesville, FL.
23. Timms, L.L. and L.H. Schultz. 1984. Mastitis therapy for cows with elevated somatic cell counts or clinical mastitis. J. Dairy Sci. 67:367-571.
24. Tyler, J.W., R.C. Wilson and P. Dowling. 1992. Treatment of subclinical mastitis. Vet. Clin. North Am. Food Anim. Pract. 8:17-35.