Introduction
Current methods of mastitis control advocate the adoption of management practices developed for mature lactating and dry cows, and include pre- and postmilking teat dipping, milking of clean and dry teats, dry cow therapy, proper use of functionally adequate milking machines, prompt treatment of clinical cases, and culling of chronically infected animals.Management practices and disease control for heifers include proper housing, adequate nutrition, artificial insemination, and vaccination against calfhood diseases, with little or no concern about mastitis. However, these animals do become infected, and in some herds, the overall mastitis level is greater than 95%, with Staphylococcus aureus causing 35% of these infections. Heifer mastitis is not to be confused with "summer mastitis", which is caused by Actinomyces pyogenes, Peptococcus indolicus, and/or Streptococcus dysgalactiae, and spread by the fly, Hydrotae irritans.
The mammary glands of heifers have traditionally been regarded as uninfected, and they are not examined until the first milking or during the first episode of clinical mastitis following calving. The greatest development of milk-producing tissue in heifers occurs during the first pregnancy, so it seems logical to protect these young animals from the harmful effects of mastitis-causing bacteria to ensure maximum future milk production.
Research Herd Studies on Heifer Mastitis
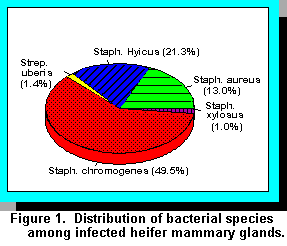 The prevalence of mastitis in unbred 10- to 12-month-old Jersey heifers at the Hill Farm Research
Station was initially evaluated in 1985 and was monitored over a 1-year period (1). At the initial
sampling, mastitis was diagnosed in 86.2% of the quarters, which was distributed among
organisms as shown in Figure 1. Of these, most infections (70.8%) were caused by the
coagulase-negative staphylococci (Staphylococcus chromogenes and Staphylococcus hyicus)
commonly called CNS. Infections caused by Streptococcus uberis were infrequent (1.47%);
however, S. aureus mastitis was much more prevalent than expected (13.0%). The vast majority of
infections by all bacterial species persisted throughout the 1-year period.
The prevalence of mastitis in unbred 10- to 12-month-old Jersey heifers at the Hill Farm Research
Station was initially evaluated in 1985 and was monitored over a 1-year period (1). At the initial
sampling, mastitis was diagnosed in 86.2% of the quarters, which was distributed among
organisms as shown in Figure 1. Of these, most infections (70.8%) were caused by the
coagulase-negative staphylococci (Staphylococcus chromogenes and Staphylococcus hyicus)
commonly called CNS. Infections caused by Streptococcus uberis were infrequent (1.47%);
however, S. aureus mastitis was much more prevalent than expected (13.0%). The vast majority of
infections by all bacterial species persisted throughout the 1-year period.Milk somatic cell counts (SCC) are considered an important parameter for assessing mammary health status in lactating cows, and it is well recognized that milk production decreases as SCC and level of mastitis increase. Thus, SCC in heifer mammary gland secretions were analyzed to measure degree of inflammation and potential reductions in future milk yield. Somatic cell counts were elevated in all infected quarters, averaging 8.1 million/mL for CNS-infected quarters and 9.2 million/mL for S. aureus-infected quarters. Histological examination of chronically infected quarters of the developing udders from two heifers revealed inflammation of the mammary parenchymal tissues characterized by an intense lymphocyte infiltration. Samples taken from the teat skin and teat canal keratin contained many species of staphylococci, suggesting these sites as potential sources of bacteria for infecting the developing mammary glands.
Heifer Mastitis in Commercial Herds
A study was then designed to determine prevalence of mastitis in breeding age and pregnant heifers in commercial herds, and to determine if intramammary treatment during pregnancy was effective in reducing how much mastitis at calving (7, 8, 9, 10). Because bacteria from the heifers' environment, flies, or from other heifers appear to colonize teat canal keratin as a prelude to intramammary infection, keratin samples were initially taken after teat end sanitization with 70% ethanol, followed by the collection of secretion samples. After collection, teats were dipped in a barrier product as a safeguard against bacteria penetrating the teat canals, which may have been more patent after the collection of keratin and the expression of mammary secretions.Results demonstrated that teat canal infections were found in 93% of heifers or 71% of teats (8). Similarly, intramammary infections were found in 97% of heifers or 75% of quarters, and 29% of heifers showed clinical symptoms of mastitis as evidenced by clots, flakes, or blood in mammary secretions. Staphylococcus aureus was isolated from 20% of all quarters and from 25% of quarters with clinical symptoms. These bacteria cause severe damage to mammary tissue, and it is known that infections are very difficult to eliminate in lactating cows. Such infections in heifers are of great concern because of the possible deleterious effect on future milk production. Examination of mammary gland tissues from seven heifers demonstrated marked inflammation in S. aureus-infected quarters, and limited development of milk secretory tissues (7).
Reasons why young dairy animals become infected with mastitis-causing bacteria are largely unknown. Nevertheless, management practices such as fly control, use of individual calf hutches to avoid suckling among calves (particularly those fed mastitic milk), and segregation of pregnant heifers from dry cows may help to prevent the development of mastitis in heifers. In addition, replacement heifers should be cultured for presence of contagious mastitis-causing bacteria prior to purchase or before entering the milking herd. Protecting the developing milk-producing tissues of heifers from bacterial invasion is important to ensure optimum future milk yield.
Antibiotic Treatment of Heifers During Pregnancy
Because of the high level of infection, elevated SCC, and prevalence of S. aureus, several heifers from each commercial herd were randomly selected to receive a one-time intramammary treatment of a penicillin/dihydrostreptomycin product. Antimicrobial susceptibility testing showed that 97% of the S. aureus isolates were sensitive to 12 antibiotics, including the product selected for treatment (10). Teat ends were sanitized, and a dry cow antibiotic containing 1,000,000 units of penicillin and one gram of dihydrostreptomycin was infused into all four quarters using the partial insertion technique. Treatments were made no less than 60 days prior to the expected calving date. To determine the effect of intramammary treatment on the level of mastitis at calving, quarters were sampled prior to treatment for determining bacteriologic and SCC status and then again at calving to determine if therapy was successful.
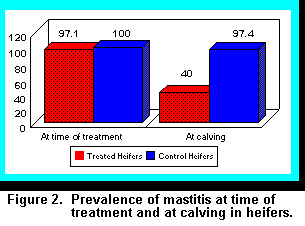 Results showed that in treated heifers, 97.1% of animals were infected at time of treatment, but at
calving, numbers of infected heifers in the treatment group were reduced to 40% (Figure 2; 9).
Only 2.9% of quarters had antibiotic residues at time of calving. Residues were limited to two
heifers treated within three weeks of calving because estimated due dates were miscalculated, but
all quarters were negative after five days. In the untreated control group, 100% of heifers were
infected at initial sampling (Figure 2), and at calving, mastitis in control heifers was reduced only
slightly to 97.4%.
Results showed that in treated heifers, 97.1% of animals were infected at time of treatment, but at
calving, numbers of infected heifers in the treatment group were reduced to 40% (Figure 2; 9).
Only 2.9% of quarters had antibiotic residues at time of calving. Residues were limited to two
heifers treated within three weeks of calving because estimated due dates were miscalculated, but
all quarters were negative after five days. In the untreated control group, 100% of heifers were
infected at initial sampling (Figure 2), and at calving, mastitis in control heifers was reduced only
slightly to 97.4%.
Staphylococcus aureus was isolated from 11 quarters of six treated heifers before antibiotic infusion (45.8%), but at calving, this organism was isolated from only one quarter of one heifer (4.2%). In the control group, 18 quarters of 10 heifers were infected with S. aureus at time of treatment (45%). At calving, six of the control heifers still had S. aureus mastitis in 11 quarters (55%). Thus, the overall level of infection was reduced 60% and that caused by S. aureus was reduced more than 90%.
In lactating cows, approximately 30% of animals or 25% of quarters with S. aureus mastitis are typically cured after antibiotic therapy. In this heifer study, 83.3% of animals or 90.9% of quarters were cured, thus antibiotic therapy in heifers was highly effective in eliminating mastitis compared with therapeutic success in lactating cows. Reasons are unclear, but the relatively small udders of heifers may have limited the microorganisms to areas of mammary tissue in which the antibiotic would be present in adequate concentrations to eliminate infection. In addition, scar tissue, common in S. aureus infections, may not have formed, which may have permitted the antibiotic to reach all infecting bacteria.
Antibiotic therapy in heifers is advantageous over treatment of lactating cows because treatment can be performed before calving, and the risk of antibiotic residues at freshening is minimal. In one commercial herd, heifers that received dry cow therapy during pregnancy produced an average of 5.5 lbs more milk per day over the first two months of lactation compared with herdmates that did not receive therapy. This amounted to a $42.12 increase per cow for the first two months of lactation, which would have paid for the cost of four mastitis tubes approximately eight-fold.
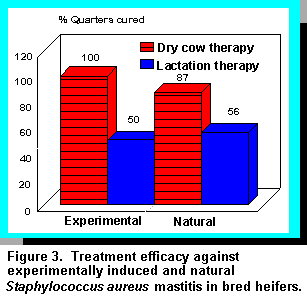 More recent studies with pregnant heifers using a cephalosporin-based nonlactating cow product
were also successful (4). Heifers either experimentally or naturally infected with S. aureus were
infused 10 weeks prepartum with one dose of a 300 mg cephapirin benzathine product and
compared with untreated S. aureus-infected controls. Results demonstrated that 100% of
experimentally induced and 87% of naturally occurring S. aureus infections were eliminated in
treated animals at the time of calving, and cured quarters remained infection-free for at least two
months into lactation (Figure 3). Quarters remaining infected at calving with S. aureus were
treated with a lactating cow product containing 200 mg cephapirin benzathine, but cure rate was
only 50 to 56%. After antibiotic infusion, SCC in infected quarters that cured decreased from 15 million/mL to 4 million/mL one week later and to 700 x 103/mL at calving. In contrast, none of the
untreated S. aureus-infected quarters had spontaneously cured by the time heifers calved. Treated
heifers in which S. aureus infections were cured produced over 10% more milk than controls
during the first two months of lactation.
More recent studies with pregnant heifers using a cephalosporin-based nonlactating cow product
were also successful (4). Heifers either experimentally or naturally infected with S. aureus were
infused 10 weeks prepartum with one dose of a 300 mg cephapirin benzathine product and
compared with untreated S. aureus-infected controls. Results demonstrated that 100% of
experimentally induced and 87% of naturally occurring S. aureus infections were eliminated in
treated animals at the time of calving, and cured quarters remained infection-free for at least two
months into lactation (Figure 3). Quarters remaining infected at calving with S. aureus were
treated with a lactating cow product containing 200 mg cephapirin benzathine, but cure rate was
only 50 to 56%. After antibiotic infusion, SCC in infected quarters that cured decreased from 15 million/mL to 4 million/mL one week later and to 700 x 103/mL at calving. In contrast, none of the
untreated S. aureus-infected quarters had spontaneously cured by the time heifers calved. Treated
heifers in which S. aureus infections were cured produced over 10% more milk than controls
during the first two months of lactation.
Generally, spontaneous cure rates for major mastitis pathogens are low. For example in a subsequent study on heifer mastitis, spontaneous cures for S. aureus and the environmental streptococci were 9% and 6%, respectively (5). Thus, treatment is required to cure such infected quarters in these young dairy animals. New infection rates in uninfected quarters receiving no therapy over the 8- to 10-week prepartum period were very low for most species of bacteria. However, new environmental streptococcal infections were quite common in uninfected, untreated heifers. Prophylactic treatment of such quarters prepartum resulted in a 93% reduction in new environmental streptococcal infections. Thus, use of nonlactating cow therapy not only is very effective in curing existing infections, it is also effective in preventing new infections.
Other Factors to Consider in Control of Heifer Mastitis
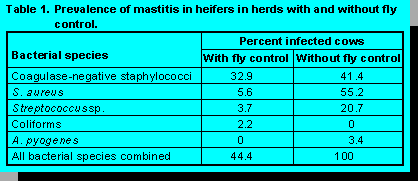
In subsequent investigations of mastitis in heifers, additional parameters were evaluated (2). For example, overall prevalence of infection was approximately twice as high in Jerseys (67.7%) compared with Holsteins (35%). Also, heifers with scabs and abrasions on the teat skin surface, presumably induced by flies, had a higher frequency of infection (70%) than normal teats (40%). Moreover, herds using some form of fly control had markedly fewer infections with environmental streptococci and S. aureus, and somewhat fewer CNS infections than those without fly control (Table 1).
 At the initial sampling during pregnancy, frequency of clinical mastitis in infected quarters among
heifers in four commercial dairies was 7.5%. At the time of calving, frequency of clinicals
increased to 24%, indicating that either the presence of new infections during the prepartum
period led to flare-ups of clinical mastitis at freshening, or chronically infected quarters exhibited
flare-ups at this time. In either case, it is apparent that clinically infected quarters in heifers should
be controlled prepartum rather than at or following freshening.
At the initial sampling during pregnancy, frequency of clinical mastitis in infected quarters among
heifers in four commercial dairies was 7.5%. At the time of calving, frequency of clinicals
increased to 24%, indicating that either the presence of new infections during the prepartum
period led to flare-ups of clinical mastitis at freshening, or chronically infected quarters exhibited
flare-ups at this time. In either case, it is apparent that clinically infected quarters in heifers should
be controlled prepartum rather than at or following freshening.
Somatic cell counts in uninfected quarters decreased from 7.6 million/mL at initial sampling to 1.5 million/mL at time of calving. In infected quarters, SCC decreased from 23.1 million/mL prepartum to 4.1 million at calving. This again indicates the need for infected heifers to be treated so that they enter the milking herd with low SCC. Additionally, the monitoring of mammary secretion characteristics demonstrated that quarters with a honey-like consistency exhibited low frequencies of infection (10%), whereas those with a thin, watery secretion exhibited a high frequency of infection (78%).
The effect of season on prevalence of infected quarters in breeding age heifers in four Louisiana dairies demonstrated that level of infection decreased through winter (55.6%), spring (42.3%), and summer (30.3%), and increased in the fall (49.6%). This trend is just the opposite of what was expected in view of the association of mastitis and the fly season in this region. However, at time of calving, prevalence of infection increased from winter (44.8%), to spring (49.6%) and summer (60.5%), and decreased in the fall (35.9%).
Some dairymen and veterinarians worry that sampling heifers for presence of mastitis may destroy the keratin plug, leading to new infections. However, studies designed to test this theory demonstrated that as long as teat ends were properly sanitized, samples were taken aseptically, and teats were dipped in a barrier type product after sample collection, there was no effect on new infection rate.
The treatment of heifers prepartum with a nonlactating cow product is advantageous because the cure rate is higher than during lactation, especially against S. aureus. There are no milk losses during therapy, the risk of antibiotic residues is minimal, SCC at calving is reduced, and milk production is increased in successfully treated cows. Treatment is indicated only in herds experiencing a high prevalence of heifers calving with clinical mastitis caused by S. aureus or S. uberis. Also, a treatment program should be developed under the supervision of the herd veterinarian. The potential for residues at calving should be considered, especially in animals that calve early. Residue testing should be carried out before mixing milk from treated animals with herd milk.
Therapy With Lactating Cow Products During the Immediate Prepartum Period
Therapy with lactating cow products has also been used successfully in heifers during the immediate prepartum period. In one study, three groups of heifers received either:
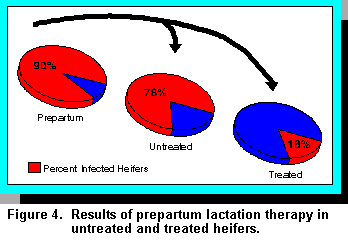 Overall, approximately 90% of heifers were infected with some type of organism prior to treatment
(Figure 4). If left untreated, 78% of heifers freshened with mastitis, but if treatment was
administered, only 18% of the heifers were infected. Some quarters did appear to cure
spontaneously. For example, in untreated control quarters, 62.2% were infected prior to calving,
and postpartum, 44.5% of the quarters remained infected. Among quarters treated with sodium
cloxacillin, 50% were infected prepartum versus 8.6% at calving. Among quarters treated with
cephapirin sodium, 70.1% were infected prepartum, and 2.1% were infected at calving. Thus, cure
rates appeared greatest for the cephapirin sodium product. However, at calving, the percentages of
samples with antibiotic residues at calving were 84.7% for cephapirin sodium compared with
17.4% for sodium cloxacillin. Three days after calving, residues were absent from quarters treated
with sodium cloxacillin, but residues were still detected in 28.2% of quarters treated with
cephapirin sodium. By 10 days, no antibiotic residues were detected in either group.
Overall, approximately 90% of heifers were infected with some type of organism prior to treatment
(Figure 4). If left untreated, 78% of heifers freshened with mastitis, but if treatment was
administered, only 18% of the heifers were infected. Some quarters did appear to cure
spontaneously. For example, in untreated control quarters, 62.2% were infected prior to calving,
and postpartum, 44.5% of the quarters remained infected. Among quarters treated with sodium
cloxacillin, 50% were infected prepartum versus 8.6% at calving. Among quarters treated with
cephapirin sodium, 70.1% were infected prepartum, and 2.1% were infected at calving. Thus, cure
rates appeared greatest for the cephapirin sodium product. However, at calving, the percentages of
samples with antibiotic residues at calving were 84.7% for cephapirin sodium compared with
17.4% for sodium cloxacillin. Three days after calving, residues were absent from quarters treated
with sodium cloxacillin, but residues were still detected in 28.2% of quarters treated with
cephapirin sodium. By 10 days, no antibiotic residues were detected in either group.
Use of Dietary Supplementation to Reduce Mastitis
Another management tool to reduce the level of infection and SCC when heifers calve and throughout lactation is through dietary supplementation with micronutrients. Diet appears to play a role in udder resistance to infection because certain nutrients affect various mammary resistance mechanisms, i.e., leukocyte function, antibody transport, and mammary tissue integrity. In one study, heifers received selenium (.3ppm/day) and vitamin E (50 to 100 ppm/day) supplementation starting 60 days prepartum (6). A selenium booster injection (50 mg) was administered 21 days prior to freshening, and the dietary supplementation was continued throughout lactation. Dietary supplementation reduced staphylococcal and coliform infections at calving by 42%. Although the rate of new infection during lactation did not differ from unsupplemented controls, duration of infection caused by organisms other than Corynebacterium bovis was reduced 40 to 50% in supplemented heifers. Clinical mastitis in supplemented heifers was reduced (57%) in early lactation and throughout lactation (32%), and mean SCC was lower. Thus, vitamin E and selenium improved udder health, and the effect of dietary supplementation was most evident at calving and in early lactation.
Additional Readings
Applied Dairy Science Course - University of Alberta:Raising Replacement Dairy Heifers
References
1. Boddie, R.L., S.C. Nickerson, W.E. Owens and J.L. Watts. 1987. Udder microflora in nonlactating heifers. Agri-Pract. 8:22-25.2. Nickerson, S.C. and R.L. Boddie. 1992. Prevalence of Heifer Mastitis in Northwest Louisiana. La Dairyman. pp. 2-3, April.
3. Oliver, S.P., M.J. Lewis, B.E. Gillespie and H.H. Dowlen. 1992. Influence of prepartum antibiotic therapy on intramammary infections in primigravid heifers during early lactation. J. Dairy Sci. 75:406-414.
4. Owens, W.E., S.C. Nickerson, P.J. Washburn and C.H. Ray. 1991. Efficacy of a cephapirin dry cow product for treatment of experimentally induced Staphylococcus aureus mastitis in heifers. J. Dairy Sci. 74:3376-3382.
5. Owens, W.E., S.C. Nickerson, P.J. Washburn and C.H. Ray. 1993. Prepartum antibiotic therapy with a cephapirin dry cow product against naturally occurring intramammary infections in heifers. Vet. Med. B. 41:90-100.
6. Smith, K.L., H.R. Conrad, B.A. Amiet and D.A. Todhunter. 1985. Incidence of environmental mastitis as influenced by dietary vitamin E and selenium. Kieler Milchwirtschaftliche Forschungsberichte. 37:482-486.
7. Trinidad, P., S.C. Nickerson and R.W. Adkinson. 1990. Histopathology of staphylococcal mastitis in unbred dairy heifers. J. Dairy Sci. 73:639-647.
8. Trinidad, P., S.C. Nickerson and T.K. Alley. 1990. Prevalence of intramammary infections and teat canal colonizations in unbred and primigravid dairy heifers. J. Dairy Sci. 73:107-114.
9. Trinidad, P., S.C. Nickerson, T.K. Alley and R.W. Adkinson. 1990. Efficacy of intramammary treatment in unbred and primigravid dairy heifers. J. Am. Vet. Med. Assn. 197:465-470.
10. Trinidad, P., S.C. Nickerson and D.G. Luther. 1990. Antimicrobial susceptibilities of staphylococcal species isolated from mammary glands of unbred and primigravid dairy heifers. J. Dairy Sci. 73:357-362.