Department of Agronomy, Pennsylvania State University,
116 Ag Science and Industry Building, University Park, PA, 16802, U.S.A.
E-mail: dbb@psu.edu
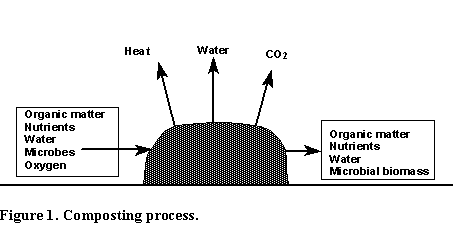
Composting is the aerobic, or oxygen requiring, decomposition of organic materials by microorganisms. In the composting process microorganisms use the organic matter containing carbon compounds, nutrients, and water as a source of energy and nutrition for growth thus breaking down the organic matter (Figure 1). In the process water vapor, heat from microbial respiration, and carbon dioxide gas are given off and the finished compost is a more stable product made up of microbial residues and the more resistant organic compounds from the raw materials. Because of the loss of water and carbon as carbon dioxide the volume of the finished compost is significantly less than that of the raw materials. There may also be some nutrient loss, particularly nitrogen (N), in the composting process. Losses of over 50% of the N in the raw materials have been observed. The magnitude of the loss of nutrients depends on the raw materials and on how the compost is made.

There are several key parameters that are important for effective composting. The ratio of the amount of carbon to the amount of N (C:N ratio) in the material being composted is very important. Carbon in the organic matter is the driving force for composting however, microbes, like all living things, also require essential nutrients like N, phosphorus (P), and potassium (K), etc. Of these nutrients N is one that usually has the greatest effect on the composting process. A material containing a large amount of carbon, but little N, i.e., a material with a high C:N ratio, may have insufficient N to support the microbial population even though there is a large amount of energy available in the organic matter. This can result in ineffective composting. At the other end of the spectrum, if there is excess N for the amount of carbon in the material i.e., a material with a low C:N ratio, the excess N will be susceptible for loss during the composting process. When this N is lost as ammonia gas, it not only is a loss of a valuable nutrient, but it can cause a strong ammonia odor problem. For effective composting the C:N ratio of the raw materials should be in the range of 20:1 to 40:1. Raw dairy manure typically has a C:N ratio of between 10:1 and 15:1. This is low for effective composting, thus it is usually necessary to mix a high C:N ratio material with the manure to achieve an acceptable C:N ratio. Common materials used for this purpose include: grain straw (C:N = 80:1), corn stover (C:N = 60:1), saw dust or wood chips (C:N = 100:1 to >1000:1) and yard waste (C:N = 20:1 to 60:1). Dairy manure which contains a significant amount of bedding may have relatively high C:N ratio (C:N = 20:1 To 30:1) and thus often can be composted without adding other materials.
A high oxygen level must be maintained in the compost pile for effective composting to occur. There are two management factors to consider here. First are the physical characteristics of the material to be composted. For effective composting oxygen must be able to diffuse throughout the compost pile to maintain an adequate oxygen supply for the microbes. The moisture content and the particle size of the raw materials are critical. Because of the small particle size and high moisture level, there is very little oxygen diffusion through a pile of raw dairy manure. Ideally for good aeration in a compost pile the moisture level of the pile should be between 50 and 60% and the particle size should average from 3 mm to 12 mm in diameter. Fortunately, the same materials that are usually used to adjust the C:N ratio of a compost mix are coarser and drier than the manure and thus will also serve as bulking agents to assure adequate aeration in the compost pile. In some cases where the material used to adjust the C:N ratio does not provide for adequate aeration, other bulking agents such as coarse wood chips may have to be added.
The second management factor in maintaining good aeration is mechanical aeration. This can be accomplished in a number of ways including: fans to force oxygen through the pile, continuous stirrers, compost turners, front-end loaders, and manure spreaders. The most common methods involve periodic mechanical turning and mixing of the compost pile using a compost turner or front-end loader. This turning mixes a fresh supply of oxygen into the pile, it loosens the compost to improve natural aeration, and it exposes fresh surfaces to the microbes. At least 5% oxygen should be maintained within the compost pile for good composting. Usually, rather than measuring the oxygen level, the temperature of the pile is used as an indicator for when a compost pile should be turned. Figure 2 shows a typical temperature pattern in a compost pile.
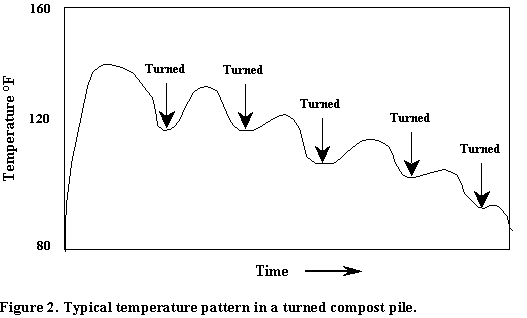
A significant drop in the temperature indicates that microbial respiration is declining due to insufficient oxygen in the compost pile. The ideal temperature in a compost pile should be between 54.4 and 60C. When the temperature drops much below this range the pile should be turned if optimum composting is to be achieved. Over time as the available carbon is used up by the microbes the peak temperature following turning will decline. When there is little increase in temperature after turning this is one indication that the process is nearing completion or that some other factor such as available carbon or a nutrient deficiency is limiting microbial activity. The frequency of turning depends on several factors. The most important are the desired completeness of the composting process and how rapidly the process will be completed. For example, for field spreading on agronomic crops a very well cured compost is not necessary and a high frequency of turning would be uneconomical. However, if the compost is being produced for sale to be used around sensitive ornamentals it would be desirable and economically viable to produce a very high quality product as rapidly as possible by frequent turning.
Often the issue of primary interest in regard to composting manure is the effect that composting has on the nutrients in the manure. There is usually some loss of nutrients in the composting process. The main mechanisms for loss are volatilization, leaching, and runoff of nutrients from the compost pile. Leaching and runoff losses can be minimized through proper site design for the compost production area. These practices will generally result in minimal P and K losses. Nitrogen losses through volatilization can be significant. The most common causes of high N volatilization losses are low C:N ratio and high compost pH. As noted earlier the ideal C:N ratio should be between 20:1 and 40:1. When the C:N ratio is below this range there is a high potential for N loss by volatilization. The ideal pH for compost is between 6.5 and 8.0. At a pH above 8.0 significant N volatilization can also occur.
In addition to loss of nutrients there are significant changes in the forms of the nutrients in the compost compared to the manure. In raw dairy manure approximately 30 to 40% of the N is in the ammonium form or forms, such as urea, that readily convert to ammonium N. This N is readily available to crops and behaves the same as similar fertilizer sources. For example, since most of this N is in the form of urea in dairy manure it has the same properties as urea fertilizer. This means that it is very soluble and available to the crops. However, since urea N is also susceptible to volatilization losses if it is not incorporated into the soil immediately following application, this fraction of N in manure is also susceptible to the same volatilization losses if the manure is not incorporated. It is usually assumed that if the manure is not incorporated immediately most of this fraction of the manure N will be lost and thus will not be available to the crop. Availability of this ammonium N fraction is estimated the same way regardless of whether it is a component of raw manure or compost. The major difference between raw manure and compost in regard to ammonium N is amount of ammonium N in these materials. Generally there is very little ammonium N in composted manure. Usually the ammonium N in compost is less than 10% of the total N.
For practical purposes the remainder of the N in manure or compost is lumped into an "organic" fraction. The availability of this fraction is much more difficult to predict because this N becomes available through microbial breakdown of the organic fraction of the manure or compost. This process is called mineralization. The N in this organic fraction will be less available than fertilizer N or than the ammonium fraction in manure or compost. Also, the release of this organic N will occur over an extended period of time with significant release of N over a period of years. Finally, it would be expected that the organic N in the compost would be less available than that in the raw manure because the easily mineralizable organic N has already been broken down in the composting process. Thus the finished compost contains a much more resistant form of residual organic N. This results in lower organic N availability from compost compared to the raw manure. For raw dairy manure it is commonly assumed that 35% of the organic N will become available in the year that the manure is spread. For compost this availability is only around 10% of the organic N in the year the compost is spread.
There will be a slow release of N as the organic matter from the manure or compost is mineralized over a period of years. Even though it is known that this will occur for many years following an application most calculations to estimate the residual contribution only go back three years. For example, in Pennsylvania we assume that the residual N contribution from dairy manure applied the previous year is 12% of the original organic N, 5% from the year before that, and 2% for dairy manure applied 3 years previous. For compost, these figures are 5, 2, and 1% of the organic N for the same 3 years respectively.
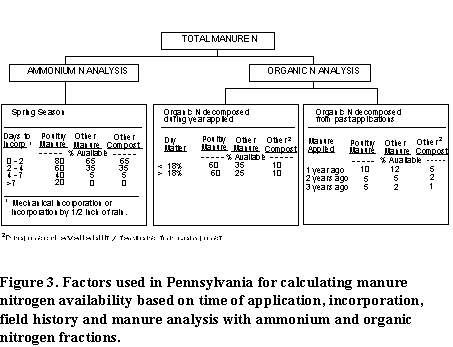
Figure 3 summarizes the factors that are used in Pennsylvania to estimate the N availability from manure and compost. This approach should be applicable in other areas, but the factors may be different due to manure characteristics, soil properties, and climatic differences. To use this approach determine if there are local factors available to make these calculations.
Less is known about P and K availability from manure and compost. Generally it is assumed that the availability of manure and compost P and K are about 80 to 100% of fertilizer P and K. Actually, preliminary work at Penn State has indicated that the immediate availability of P from compost may be higher than from manure. Other work has also indicated that the long term effects of manure application may result in higher P availability from manure than from fertilizer. These are areas that need further study.
One concern that has arisen related to low N availability from compost is the long term consequences of the large amounts of N that must be applied to supply adequate available N to meet crop needs. For example, using the availability factors from Pennsylvania and a typical dairy manure compost analysis, a farmer would need to apply around 250 t of compost per hectare and incorporate it immediately to meet the available N needs of a 10 t/hectare corn crop. At this rate approximately 920 kg of total N would be applied! Eventually all of this N will be released and raises a concern about the ultimate fate of this N. This is especially a concern if this application is made on a routine basis. In addition to all of this N, about 600 kg of P2O5 and 730 kg of K2O would be applied annually at this application rate. Compared to a typical nutrient recommendation for corn of 180 kg N/h, 65 kg P2O5 /h, and 50 kg K2O /h this represents a large excess of nutrients being applied.
Composting of manure has been proposed to play a role in manure management programs designed to protect water quality. As discussed above, it is known that the biological activity involved in composting changes the nutrient availability of the material and in some cases will reduce the nutrient content. However, the major role of composting may be in helping to alleviate some of the on-farm manure nutrient excess problems. Composting contributes to this by facilitating manure movement from a farm with an excess to a farm with a deficit of nutrients. There are several concerns that compost can address in this situation. Composting reduces the amount of material to be transported. This is critical because one of the main limitations to moving manure is the high cost of moving the low analysis, high moisture, bulky material. Composting reduces the hazards from transporting weeds and diseases because of the high temperatures involved in the composting process. Odors are usually reduced by composting. Finally, composting improves the perception that the public has about the material. There is much greater public acceptance of compost than raw manure, regardless of the properties of either material. In fact, composted manure is a marketable product in some cases that can result in significant farm income. Thus, composting manure could play a role in dealing with the potential environmental problems related to manure nutrients.