 Figure 1. Changes in milk production in USA
during the last century.
Figure 1. Changes in milk production in USA
during the last century.
Dairy and Swine Research and Development Centre,
Agriculture and Agri-Food Canada, P.O. Box 90,
2000 Road 108 East, Lennoxville, QC, J1M 1Z3 Canada
E-mail: girardch@em.agr.ca
Take Home Messages
Introduction
Synthesis of B-complex vitamins by the ruminal microflora was demonstrated a long time ago and it was observed that the concentration of most of the B-complex vitamins in ruminal digesta increases in cows fed readily available carbohydrates (64). Moreover, it was demonstrated that, even when a vitamin-free diet is fed to cattle, the concentrations of B-complex vitamins in the ruminal digesta do not vary and are similar to the concentrations observed in cows fed normal diets (60). Therefore, as requirements for B-complex vitamins are defined as the smallest amount of these vitamins to include in the diet to avoid deficiency symptoms, the conclusion was that B-complex vitamins are synthesized in amounts adequate to sustain normal gestation and lactation in dairy cows, except under certain conditions such as cobalt deficiency or some dietary intoxications (4, 53, 74). However, the 1989 NRC Requirements (74) acknowledge that high-producing cows in early lactation could benefit from dietary supplements of niacin.
With some exceptions, the statement of Theiler (1915, cited by 61) still applies to dairy cow nutrition:
"... vitamin requirements of cattle are so low that they may even be covered indirectly by synthesis carried out by the extensive bacterial flora in the intestine".
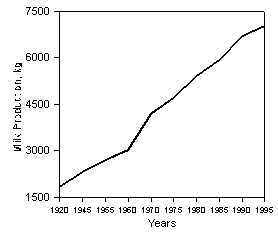
However, some questions could be raised about this statement. The first question is about the definition of requirements. For example, requirements for protein and energy are not submitted to the "minimalist" definition of vitamin requirement. They are defined as the quantity required to maximize health and productivity. Requirements for B-complex vitamins need to be reevaluated in a similar manner. Moreover, studies on B-complex vitamin requirements for dairy cows are scarce and most were done in the 40's and 50's. During the last half century, milk yield has increased dramatically in the USA (Figure 1) and in Canada (79). In order to sustain these levels of milk production, nutritionists balance diets to cover the augmented needs for energy and protein, but little attention has been given to the requirements for B-complex vitamins. However, it is unlikely that the ruminal microflora adapted its own production of B-complex vitamins in order to fulfill the increased dairy cow requirements. Therefore, the usual statement that dairy cows have no need for dietary supplements of B-complex vitamins has to be challenged and the requirements must be reviewed in the light of current levels of milk production.
 Figure 1. Changes in milk production in USA
during the last century.
Figure 1. Changes in milk production in USA
during the last century.
The aim of this paper is to review the dietary requirements for B-complex vitamins in the context of optimizing the health and productivity of today's high-producing dairy cows.
The B-Complex Vitamins
The vitamins are defined as a group of organic compounds present in minute amounts in feedstuffs. They do not have an energy value on their own, but they are essential to normal metabolism and their absence from the diet causes deficiency symptoms. However, some vitamins, such as vitamins C, D, and niacin deviate from this definition because, under some conditions and for specific species, they can be synthesized in the animal tissues.
Traditionally, the B-complex vitamins are thiamin (B1), riboflavin (B2), niacin (B3 or PP), pantothenic acid (B5), pyridoxine (B6), biotin (B8), folic acid (B9) and vitamin B12. Choline is sometimes classified as a B-complex vitamin even though it does not satisfy the definition of a vitamin. Choline can be synthesized in the liver and is required in the body in greater amounts than vitamins. Moreover, it apparently functions as a structural constituent rather than as a coenzyme.
The classification of B-complex vitamins is not based on chemical or metabolic similarities. The B-complex vitamins are a group of very heterogenous chemical compounds completely different from each other in their chemical structure, physico-chemical properties, as well as their metabolic roles. In fact, they are so different from each other that knowledge of one B-complex vitamin does not give any information on the others. They are classified together only for historical reasons. At the beginning of the century, the fat-soluble vitamins and vitamin C were isolated, whereas a group of water-soluble vitamins remained undifferentiated. Afterwards, as they were isolated from the same sources, liver and yeast, and because, in humans, deficiency in more than one vitamin B was generally observed, these different vitamins were considered as a group, the B-complex vitamins.
Niacin
Chemical Structure and Metabolic Roles
Niacin has the least complex structure of the B-vitamins. Nicotinic acid (C6H5O2N) and niacinamide (C6H6ON2) possess the same vitamin activity. Niacin was isolated in 1867, but its metabolic importance was not discovered until 70 years later when its role in the prevention of pellagra was demonstrated. In 1945, it was demonstrated that tryptophan could also prevent pellagra and a few years later, synthesis of nicotinic acid from tryptophan was demonstrated in rats. Consequently, as niacin can be synthesized from tryptophan, it does not strictly adhere to the definition of a vitamin.
Niacin is involved in many metabolic reactions in the coenzyme forms of nicotinamide, NAD and NADP. These enzymes are especially important in metabolic reactions that provide energy to the animal. They are involved in the metabolism of carbohydrates (glycolysis, Krebs cycle), lipids (glycerol anabolism and catabolism, oxidation and synthesis of fatty acids, steroid synthesis), and proteins (degradation and synthesis of some amino acids, oxidation of carbon chains through the Krebs cycle).
Requirements and Sources
It is generally accepted that, as for all the other water-soluble vitamins, dairy cow requirements for niacin are met from the diet, synthesis by ruminal microflora and specifically for niacin, by conversion of tryptophan to niacin by the animal.
Niacin requirements seem to be increased in high producing dairy cows in early lactation when the animals are in energy deficiency. Moreover, during this period of high demand, synthesis of niacin from tryptophan is negligible because available tryptophan is required for milk protein synthesis.
The best sources of niacin are animal and fish by-products, distiller's grains, yeast, and some oilseed meals. Pasture can provide a fair amount of niacin, but niacin in cereals is generally not available to the animal.
Effects on Ruminal Microflora
In a large number of in vitro and in vivo studies, niacin increases the production of ruminal microbial protein (50, 51, 75, 77, 78, 85, 86) and propionate (31, 50, 77). Ottou and Doreau (75) observed an increase in the in vitro production of total and individual volatile fatty acids whereas in vivo, supplementary niacin increased ruminal butyrate concentration, but did not change total volatile fatty acids concentrations (24). The effects of supplementary niacin on ruminal fermentation are frequently attributed to an augmentation in the number of ruminal protozoa (22, 24, 29, 31, 50) because ruminal protozoa need an exogenous supply of niacin. Nevertheless, some studies do not report any effect of supplementary niacin on ruminal microbial populations or fermentation characteristics (1, 13, 62).
Metabolic Effects of Supplementary Niacin
Acute doses of niacin decrease plasma concentrations of non-esterified fatty acids (NEFA) and ketone bodies in early lactation dairy cows suffering from subclinical or clinical ketosis. However, this decrease is followed by a dramatic increase in the concentration of these metabolites 24 h after cessation of niacin supplementation (91, 92, 93). A similar effect on the pattern of plasma concentrations of NEFA and ketone bodies is observed after ingestion of a single large dose of niacin (120 mg) by non ketotic cows (55). However, a daily supplement of 12 g of niacin administered during 7 days to cows suffering from subclinical or clinical ketosis rapidly decreases plasma concentrations of NEFA and ketone bodies while increasing plasma concentrations of glucose and maintaining the concentrations of these metabolites within a normal range (33). These effects are due to the antiketogenic and antilipolytic actions of niacin. This vitamin decreases lipolysis in liver adipocytes and then reduces fat mobilization (88).
Many studies report that supplementary niacin given to healthy cows during the peripartum period and in early lactation decreases plasma concentrations of NEFA and ketone bodies while increasing plasma concentrations of glucose (26, 30, 49, 54, 95). However, in other studies, no effect was observed on these metabolites (6, 9, 25, 43, 55, 71, 87).
In mid- and late-lactation, supplementary niacin has no effect on plasma concentrations of NEFA, ketone bodies, and glucose ( 13, 14, 16, 29, 63, 67).
Supplementary Niacin and Milk Performance of Dairy Cows
Effects of supplementary niacin on milk production of dairy cows are variable. Summarizing these studies, it appears that feeding 6 mg of niacin per day during the peripartum period and in early lactation increases, or tends to increase, milk production (15, 26, 43, 54, 55, 56, 62, 73, 78). Horner et al. (51) and Cervantes et al. (14) also reported an increase in milk production when supplementary niacin is fed past the lactation peak. From all these studies, the average increase in milk production was 6% and varied from 1.6 to 10%. However, other studies observed no effect on milk production when supplementary niacin was given in early (6, 9, 25, 49, 71), mid- (7, 95), or late-lactation (63). In most studies, supplementary niacin does not modify milk composition.
An increase in feed intake of 5 to 10%, required to sustain an increased milk production, is frequently observed following ingestion of dietary supplements of niacin (14, 62, 78).
Metabolic and production responses to supplementary niacin vary among studies depending on stage of lactation and the metabolic status of the dairy cow. Supplementary niacin modifies metabolic and production responses when given to dairy cows during the peripartum period and in early lactation when body reserves are being mobilized. In fact, the most beneficial effects of niacin are observed in high-producing cows in early lactation when their feed intake is insufficient to cover requirements for milk production and when they have sufficient body reserves.
Biotin
Chemical Structure and Metabolic Roles
The chemical structure of biotin, C10H16O3N2S, includes a sulfur atom in its ring and a transverse bond across the ring. Only one isomer of this molecule is biologically active. The existence of biotin has been suspected since 1916 when the toxic effects of raw egg white could be overcome by an unidentified factor present in certain foods i.e., egg yolk and liver. The chemical structure and the properties of biotin were only identified in 1942 and it was not until 1970 that the importance of biotin in farm animal nutrition was known. Most of the research on biotin has been conducted in poultry and pigs.
In ruminants, biotin is essential to the activation of three carboxylases, enzymes catalyzing the incorporation of CO2. These enzymes are 1) acetyl-CoA carboxylase (acetyl-CoA malonyl-CoA), essential to the synthesis of fatty acids; 2) propionyl-CoA carboxylase (propionyl-CoA methylmalonyl-CoA), allows the entry of propionate, odd-chain fatty acids, and some amino acids, such as valine, isoleucine, methionine, and threonine in the Krebs cycle, and 3) pyruvate carboxylase (pyruvate oxaloacetate), allows the entry of lactic acid and some amino acids, such as alanine and glycine, in the Krebs cycle.
Sources and Requirements
Although it has not been demonstrated, it seems logical that the demand for biotin is high at a time when these three enzymes are very active, during the peripartum period and the first weeks of lactation when the energy demand is high, and the gluconeogenesis reaches its maximum.
Cereals are poor sources of biotin, but forages, because they are ingested in large quantities, could be a relatively good source of biotin. Alfalfa meal as well as oilseed meals are very rich sources of biotin.
Ruminal Microflora
Diets high in starch and other fermentable carbohydrate sources which are supplemented with urea result in increased concentrations of biotin in ruminal digesta (11, 64). However, it has not been clearly demonstrated if this augmentation is related to an increased synthesis or a decreased utilization of biotin by the ruminal microflora. Moreover, in these two studies data were not present on the availability of the additional biotin to the animal.
It has been demonstrated that the cellulolytic activity of ruminal microflora requires the presence of biotin. Cellulose digestion and production of volatile fatty acids decrease when the in vitroruminal fermentation medium is low in biotin (58, 70).
Biotin and Dairy Cow Performance
The few studies on dairy cow requirements for biotin demonstrate that biotin is involved mainly in hoof health which is similar to what has been observed in pigs (12).
A daily dietary supplement of 20 mg of biotin decreased the frequency of lameness related to horn weakness by 50%, but had no effect on milk yield and milk composition or reproductive performance (20). In a controlled study using a small number of dairy cows, a daily dietary supplement of 20 mg of biotin given over a 5 month period increased quality and tensile strength of sole and heel horn, but it took 10 months before coronary horn quality was improved (82). In a field trial conducted on 160 cows with foot lesions, biotin did not accelerate the healing process, but it increased the rate of new horn formation (52). Frequency and severity of sole ulcers and/or heel erosion are also decreased as a result of long-term biotin supplementation (23, 45, 47, 48).
Biotin has no direct effect on dairy cow performance in these studies, but hoof health could indirectly affect dairy cow productivity. Indeed, epidemiological studies link lameness frequency with an increase of the number of days open and the number of services per conception (18, 65) and in some cases, with a decrease in milk production (19).
In herds with a high frequency of foot lesions, daily dietary supplements of biotin given during a period of several months might substantially improve horn quality and consequently reduce foot lesions and frequency of associated problems.
Folic Acid
Chemical Structure and Metabolic Roles
The molecular structure of folic acid was elucidated in 1945. The molecule of folic acid (pteroylmonoglutamic acid) is constituted of three different parts: 1) a pteridine nucleus, 2) a paraaminobenzoic moiety, and 3) one glutamic acid molecule; this is the synthetic form of the vitamin. However, due to changes in the level of reduction of the pteridine nucleus (dihydrofolate or tetrahydrofolate), the addition of different one-carbon radicals (methyl, formyl, methenyl, etc.), as well as the presence of a variable number of glutamic acid molecules, more than 100 biologically active forms of this vitamin have been identified. The biologically active forms of the vitamin are called folates.
Folic acid is a donor of one-carbon units and it is involved in numerous reactions. Folic acid is involved in amino acid metabolism: 1) glycine catabolism, 2) histidine catabolism, 3) glycine-serine interconversion, and 4) methionine synthesis. It is also involved in protein metabolism as it provides a one-carbon unit for the synthesis of formylmethionine; the presence of a formylmethionine on a t-RNA is essential to the induction of protein synthesis. Folic acid is also necessary for purine and pyrimidine synthesis, essential constituents of RNA and DNA.
Requirements and Sources
Brewer's grains, alfalfa meal, and soybean seeds are good sources of folic acid, whereas cereals are poor sources.
Given its metabolic roles, folic acid is absolutely essential to cell division and growth as well as to protein synthesis. All these conditions are highly augmented during gestation and lactation (5, 66, 68, 69, 76). Synthesis of new tissues such as the foetus, foetal membranes, mammary gland, and milk protein synthesis increases the utilization of folic acid. Consequently, it is logical to believe that requirements for folic acid are high in dairy cows which are lactating and/or pregnant for most of their life.
Under controlled conditions, serum concentration of folates is a good indicator of folate status (34). It is also true in dairy cows if feeding and milking schedules do not vary (89). Total serum folates of dairy cows decrease by 40% from 2 months after calving to calving (38; Figure 2). Other studies observed a similar phenomenon. Non-gestating cows have serum concentrations of folates which are greater than those of gestating cows (3). Tremblay et al. (89) also observed that serum concentration of folates is lower in cows at one month prepartum than at two months postpartum. This dramatic fall of serum folates reflects a huge demand for folic acid as was demonstrated in multiparous cows, in which tissue demand for folic acid is higher two months before calving than three weeks after calving (36).
Ruminal Microflora
According to Kon and Porter (61), folates in ruminal contents are not related to dietary supply of folic acid. However, in a later study, it was observed that concentration of folates in ruminal content is higher in steers fed a high grain diet (70% barley - 30% timothy hay) than in those fed a high forage diet (30% barley - 70% timothy hay) (40). The higher concentrations in the ruminal content of steers fed the high-grain diet could be due to increased folate synthesis by ruminal microflora as observed in cattle fed readily fermentable carbohydrates (46, 64). However, the concentrations in ruminal content of steers fed a high forage diet could be decreased further by increased utilization of folates by microorganisms involved in the degradation of fibrous substrates as observed in vitro by Allison (2) and Blanchart et al. (10).
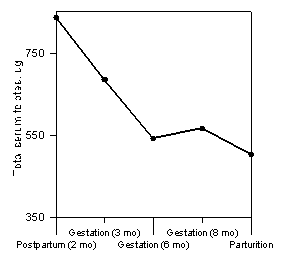 Figure 2. Changes of total serum folates
during gestation and lactation of multiparous
dairy cows (Adapted from Girard et al.,
1989).
Figure 2. Changes of total serum folates
during gestation and lactation of multiparous
dairy cows (Adapted from Girard et al.,
1989).
Dietary supplements of folic acid have no effect on total tract digestibility of DM, ADF, NDF, or protein. Ruminal concentrations of acetate and butyrate are also not affected by folic acid supplementation, but ruminal propionate concentrations are elevated (17). They have no effect on ruminal microbial protein (40).
Dietary supplements equivalent to 0.05 and 0.5 mg of folic acid per kilogram of body weight do not increase the quantity of folic acid reaching the duodenum (96). However, amounts of folic acid greater than 0.5 mg per kilogram of body weight increase serum concentrations of folates (39). Dietary supplements of folic acid, 2 mg of folic acid per kilogram of body weight, increase both concentrations of folates in the ruminal content and in serum of steers (40).
Supplementary Folic Acid and Dairy Cow Performance
Following these indications that the tissue demand for folic acid is high in dairy cows, two experiments were conducted to study the effects of supplementary folic acid on annual performance.
In the first experiment, folic acid was given by intramuscular injections (0 or 160 mg of folic acid), administered weekly from 45 days of gestation to 6 weeks after calving. Cows were fed a diet based on grass silage. Supplementary folic acid increased placental and colostral transfer of folates to the calf by 24% and 54%, respectively. During the period from 45 days of gestation until drying off, supplementary folic acid increased transfer of folic acid in milk, tended to increase milk protein content and increased milk yield by 1.5 kg/d. After calving, injections of folic acid had no effect on milk folates or milk yield. Supplementary folic acid had no effect on milk protein content in primiparous cows, but they drastically increased milk protein content of multiparous cows (3.23 vs. 3.51%). Injections of folic acid did not modify dry matter intake of cows (41).
In the second experiment, dietary supplements of 0, 2, and 4 mg of folic acid per kilogram of body weight were used daily from 4 weeks before the expected time of calving until 305 days of lactation. Cows were fed a high-concentrate diet. Dietary supplements of folic acid increased serum folates indicating that folic acid was efficiently absorbed. Milk folates were also increased by supplementary folic acid. This finding could be of interest for human health given the present interest in the use of folic acid in the prevention of neurological birth defects (81, 94), coronary heart diseases and strokes (35, 72), and cancer (42, 57). In contrast to the previous experiment milk composition was not modified by the dietary supplements of folic acid with the exception of a decrease in non protein nitrogen content in the milk of multiparous cows. The decrease in non protein nitrogen in milk could be an indication that multiparous cows fed supplementary folic acid used nitrogen more efficiently than unsupplemented cows. Moreover, milk protein yield of these cows tended to be increased by ingestion of supplementary folic acid. This was due to the effect of folic acid on milk production as observed in the previous experiment. Milk yield of first lactation cows was not significantly changed by folic acid, whereas in multiparous cows milk yield increased linearly with increasing level of folic acid supplementation. Multiparous dairy cows fed daily 0, 2, or 4 mg of folic acid per kilogram of body weight produced 8284, 8548, and 8953 kg of milk, respectively, during a 305-d lactation period. Multiparous cows receiving 4 mg of folic acid per kilogram of body weight daily produced 2.2 kg/d more milk than control cows during their lactation (37). Ingestion of dietary supplements of folic acid did not modify dry matter intake of cows (unpublished data). Observations on the effects of folic acid on feed intake, non protein nitrogen in milk, and milk production of multiparous cows could be an indication that folic acid improves the efficiency of nutrient utilization in these animals.
In conclusion, according to these two studies, supplementary folic acid increases milk production of multiparous cows. However, the response in milk protein differed between the two studies. This discordance between the two studies could be due to the composition of the diets used. In fact, in the first experiment, cows were fed a diet based on grass silage, promoting synthesis of vitamin B12 from dietary cobalt and in which the first calculated limiting amino acid for milk synthesis was methionine. In the second experiment, the high-concentrate diet used decreased the synthesis of biologically active vitamin B12 (44) and the calculated supply in lysine and methionine were less than the recommended levels (80, 83, 84). The decreased availability of vitamin B12 in the second experiment could limit the utilization of folic acid by the cow. This topic will be further discussed in the next section. Moreover, folic acid is known to promote synthesis of methionine in mammalian tissue. Consequently, an increased supply of folic acid could augment the amount of methionine available for milk synthesis. This might explain the increase in milk protein content observed in the first experiment in which the first limiting amino acid was methionine. However, in the second expriment, even if folic acid partially relieved the lack of methionine, milk protein could not be increased because lysine was the first-limiting amino acid in the diet. Other experiments are in progress to verify these two hypotheses and to determine which types of diets will optimize the effects of supplementary folic acid on milk production and composition.
Vitamin B12
Chemical Structure and Metabolic Roles
Vitamin B12 was the last vitamin discovered, in 1948. Vitamin B12 differs from the other B-complex vitamins. This vitamin has the highest molecular weight and the most complex chemical structure of all vitamins. Moreover, the molecule of vitamin B12 contains a cobalt atom. The main molecules with biological activity are cyanocobalamin (-CN), hydroxocobalamin (-OH), methylcobalamin (-CH3), nitrocobalamin (-NO2) and adenosylcobalamin (-5'-desoxyadenosine).
Vitamin B12 is not synthesized by plants as the other B-complex vitamins; this vitamin is produced only by bacterial synthesis. As one atom of cobalt is part of the molecule of vitamin, it is assumed that ruminant requirements for vitamin B12 equal ruminal bacteria requirements for cobalt. This characteristic of the vitamin to result only from bacterial synthesis also explains the presence of several molecules with a chemical structure close to that of vitamin B12, but without its biological activity. Those molecules are called "vitamin B12 analogues, pseudo-vitamin B12 or vitamin B12-like factors". They are probably intermediate molecules formed during the vitamin B12 biosynthesis. They are present in substantial amounts in sewage, manure, ruminal contents, and residues from fermentation.
Two enzymes are vitamin B12-dependent. The first one is the methionine synthase, essential for the transfer of one-carbon unit from the methylated form of folic acid to an amino acid, homocysteine to form methionine. A lack of vitamin B12 decreases the quantity of methionine available for the animal and blocks the utilization of folic acid by the tissues. The methylated form of folic acid is the form in circulation in serum. However, the molecule must lose its methyl group to be used by the cells. In the absence of vitamin B12, there is no demethylation of folic acid and its methylated form is trapped in the serum, leading to a secondary deficiency in folic acid.
The second enzyme that is vitamin B12-dependent is the methylmalonyl-CoA mutase. This enzyme transforms the inactive isomer of methylmalonyl-CoA to its active isomer. The active form will be transformed to succinyl-CoA and then will enter the Krebs cycle. The methylmalonyl-CoA is the result of degradation of odd-chain fatty acids, some amino acids (valine, isoleucine, methionine, threonine) and propionate. In dairy cows methylmalonyl-CoA mutase, is very important in the metabolism of propionate.
Requirements and Sources
Vitamin B12 is more potent than the other B-complex vitamins. The requirements of most species are met by only a few micrograms of vitamin B12 per kilogram of feed. Moreover, vitamin B12 is the only water-soluble vitamin stored in tissues for a relatively long period of time. Consequently, deficiency symptoms appear only after a long period on a deficient diet. However, given the large amounts of propionate synthesized by the ruminal microflora, a lack of vitamin B12 decreases the utilization of propionate by the animal, its accumulation in blood leading rapidly to a decrease of feed intake. The importance of the propionate metabolism explains the higher ruminant requirements for vitamin B12 as compared to monogastric animals. However, it seems that cattle are less sensitive to a lack of vitamin B12 than sheep (59).
Feedstuffs of animal origin (i.e., meat or fish meal), could provide some vitamin B12. However, in ruminants, vitamin B12 is mainly supplied by ruminal microflora synthesis if dietary cobalt is adequate. Bacterial synthesis of vitamin B12 is influenced by the diet composition. High-concentrate diets increase propionate production (8) and the demand for vitamin B12 for propionate metabolism (27). These diets also stimulate the bacterial production of vitamin B12analogues (44, 90). These analogues are absorbed, but they are not taken up by the liver or mammary gland (90). Consequently, in dairy cows fed high-concentrate diets the demand for vitamin B12 is increased while the supply is decreased.
Vitamin B12 and Dairy Cow Performance
Most of the research on the effect of vitamin B12 on dairy cow performance is in relation to a theory that explains the "low milk fat syndrome" observed in cows fed high-concentrate diets. According to this theory, methylmalonic acid might accumulate as a consequence of the increased propionate production and decreased vitamin B12 status due to high-concentrate diets. High concentrations of methylmalonic acid might inhibit fatty acid synthesis (32). Unfortunately, subsequent works failed to observe an increase in milk fat due to supplements of vitamin B12 (21, 28, 32). However, some of these studies reported a relation between an improvement of vitamin B12 status and an increase of milk yield, mainly in early lactation (27, 28). Girard and Matte (unpublished data) also observed that intramuscular injections of vitamin B12 increased, although not significantly, milk production in primiparous cows fed a diet adequate in lysine and methionine and supplemented with folic acid; cows supplemented with vitamin B12 produced 2.4 kg/d more milk from 25 to 125 d of lactation, 28.4 vs 30.9 kg/d. Injections of vitamin B12 increased yields of milk solids by 12% (3.4 vs 3.8 kg/d), milk fat by 16% (847 vs 983 g/d) and milk non-fat solids by 12% (2.5 vs 2.8 kg/d) (Girard and Matte, preliminary unpublished data).
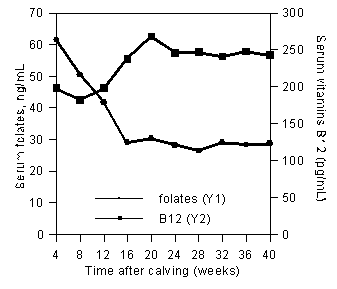 Figure 3. Serum concentrations of
folates and vitamin B12 in dairy cows
fed 4 mg of folic acid per kilogram of
body weight from 4 weeks before the
expected time of calving to 305 d of
lactation.
Figure 3. Serum concentrations of
folates and vitamin B12 in dairy cows
fed 4 mg of folic acid per kilogram of
body weight from 4 weeks before the
expected time of calving to 305 d of
lactation.
A lack of vitamin B12 might also explain, at least partially, the absence of response of milk protein content to supplementary folic acid when the cows are fed a high-concentrate diet (37). Indeed, in this experiment, serum concentrations of vitamin B12 were very low in early lactation, whereas serum concentrations of folates in supplemented cows reached very high values. Later in lactation, serum folates decreased while serum vitamin B12 increased (Figure 3). The results could be an indication that utilization of supplementary folic acid by the cow tissues was restricted in early lactation, folic acid being "trapped" in serum by a lack of vitamin B12.
Metabolism of vitamin B12 in dairy cows is still not well understood. More research is necessary to define the optimal vitamin B12 supply for dairy cows, especially when they are fed a high-concentrate diet.
Conclusion
It is generally accepted, in dairy cow nutrition, that requirements for B-complex vitamins are fulfilled by the diet and synthesis by the ruminal microflora. Studies reported in the present paper demonstrate, however, that in contrast to this actual belief, the supply in B-complex vitamins is not always sufficient to maximize health and productivity of dairy cows. Under some circumstances, the supply of one or more of these vitamins may not meet the dairy cow requirements and thus dietary supplements of these vitamins would have a beneficial effect.
Dairy cow nutrition has changed drastically during the last decade. It is no longer sufficient to only calculate the amounts of crude protein, energy, fat-soluble vitamins, and minerals to balance a dairy cow ration. The full expression of the improved genetic potential of dairy cows now requires that we take into account the proportion of ruminally degradable protein and carbohydrates as well as the requirement for essential amino acids. At the present time, very little information is available on the interactions between major and minor nutrients, such as B-complex vitamins. This lack of knowledge might explain the variability of the response of dairy cows to similar dietary treatments when comparing different studies. Research is necessary to determine the influence of physiological state, milk yield, and diet on the requirements for B-complex vitamins. Obviously, requirements for B-complex vitamins need to be reevaluated to optimize health and productivity of dairy cows.
References
1. Abdouli, H. and D.M. Schaefer. 1986. Effects of two dietary niacin concentrations on ruminal fluid free niacin concentration, and of supplemental niacin and source of inoculum on in vitro microbial growth, fermentative activity and nicotinamide adenine dinucleotide pool size. J. Anim. Sci. 62: 254-262.
2. Allison, M.J. 1965. Nutrition of rumen bacteria. In: Physiology of Digestion in Ruminant. Eds. R. W. Dougherthy, R. S. Allen, W. Burroughs, N. L. Jacobson and A. D. McGilliard. Butterworth, Washington, pp. 369-377.
3. Arbeiter, V.K. and W. Winding. 1973. Folatbestimmungen im Serum von Rindern mit besonderem Bezug auf die Fruchtbarkeit. Wien. Tierärztl. Mschr. 60: 323-326.
4. ARC. 1980. The nutrient requirements of ruminant livestock. Commonwealth Agricultural Bureaux. Slough, England.
5. Bailey, L.B. 1990. Folate status assessment. J. Nutr. 120: 1508-1511.
6. Ballenger, A.D. and D.L. Palmquist. 1990. Energy balance and blood plasma metabolites of first lactation cows supplemented with MegalacR and niacin. J. Dairy Sci. 73 (Suppl. 1): 219.
7. Batallas, C.E., Boman, R.L., Dawson, D.P. and M.J. Arambel. 1991. Effects of feeding a high level of rumen protected fat with bypass protein with or without niacin on milk production response in the early to mid lactation Holstein. J. Dairy Sci. 74 (Suppl. 1): 253.
8. Bauman, D.E., C.L. Davis, and H.F. Bucholtz. 1971. Propionate production in the rumen of cows fed either a control or high-grain, low-fiber diet. J. Dairy Sci. 54: 1282-1287.
9. Bernard, J.K., J.D. Quigley III, H.H. Dowlen and K.C. Lamar. 1995. Supplemental niacin and heat-treated whole soybeans for Jersey cows during early lactation. J. Dairy Sci. 78: 2016-2023.
10. Blanchart, G., M. Durand, J.L. Barry, M. Bouiller-Oudot and J.P. Jouany (1989) Intérêts et limites des fermenteurs à flux semi-continu de type Rusitec dans l'étude des fermentations du rumen. Ann. Zootech. 38: 285-314.
11. Briggs, M.H., T.W. Heard, A. Whicroft and M.L. Hogg. 1964. Studies on urea-fed cattle. 2. Rumen levels of B vitamins and related coenzymes. Life Sci. 3: 11-14.
12. Brooks, P.H. 1986. The role of biotin in intensive systems of pig production. InProceedings of the Sixth International Conference on Production Disease in Farm Animals. Belfast, Northern Ireland.
13. Campbell, J.M., M.R. Murphy, R.A. Christensen and T.R. Overton. 1994. Kinetics of niacin supplements in lactating dairy cows. J. Dairy Sci. 77: 566-575.
14. Cervantes, A., T.R. Smith and J.W. Young. 1996. Effects of nicotinamide on milk composition and production in dairy cows fed supplemental fat. J. Dairy Sci. 79: 105-113.
15. Chemillier, J. 1986. Effets positifs de la niacine en début de lactation. Production laitière moderne. 147: 61-64.
16. Chilliard, Y. and J.F. Ottou. 1995. Duodenal infusion of oil in midlactation cows. 7. Interaction with niacin on responses to glucose, insulin, and -agonist challenges. J. Dairy Sci. 78: 2452-2463.
17. Chiquette, J., C.L. Girard and J.J. Matte. 1993. Effect of diet and folic acid addition on digestibility and ruminal fermentation in growing steers. J. Anim. Sci. 71: 2793-2798.
18. Collick, D.W., W.R. Ward and H. Dobson. 1989. Associations between types of lameness and fertility. Vet. Rec. 125: 103-106.
19. Coulon, J.B., F. Lescourret and A. Fonty. 1996. Effect of foot lesions on milk production by dairy cows. J. Dairy Sci. 79: 44-49.
20. Cooke, B.C. and P.E. Brumby. 1982. Biotin - a dairy herd feeding trial. Pages 21 - 26 InHurstel, O., Roberts, C. J., D. G. Baggott, B. C. Cooke and P. E. Brumby. Biotin in Ruminant Nutrition. Proceedings of the Roche Vitamin Symposium. London, November, 1982.
21. Croom, W.J., Jr., A.H. Rakes, A.C. Linnerud, G.A. Ducharme and J.M. Elliot 1981. Vitamin B12 administration for milk fat synthesis in lactating dairy cows fed a low fiber diet. J. Dairy Sci. 64: 1555-1560.
22. Dennis, S.M., M.J. Arambel, E.E. Bartley, D.O. Riddell and A.D. Dayton. 1982. Effect of heated or unheated soybean meal with or without niacin on rumen protozoa. J. Dairy. Sci. 65: 1643-1646.
23. Distl, O. and D. Schmid. 1994. Einflu einer Zufütterung von Biotin auf die Klauenform, -Härte und -Gesundheit bei Milchkühen. Tierärztl. Umschau. 49: 581-588.
24. Doreau, M. and J.F. Ottou. 1996. Influence of niacin supplementation on in vivo digestibility and ruminal digestion in dairy cows. J. Dairy Sci. 79: 2247-2254.
25. Driver, L.S., R.R. Grummer and L.H. Schultz. 1990. Effects of feeding heat-treated soybeans and niacin to high producing cows in early lactation. J. Dairy Sci. 73: 463-469.
26. Dufva, G.S., E.E. Bartley, A.D. Dayton and D.O. Riddell. 1983. Effect of niacin supplementation on milk production and ketosis of dairy cattle. J. Dairy Sci. 66: 2329-2336.
27. Elliot, J.M. 1979. Propionate metabolism and vitamin B12. In Digestive Physiology and Metabolism in Ruminants. Eds. Y. Ruckebush and P. Thivend. AVI Publishing Co., Inc. Westport, Co, pp. 485-503.
28. Elliot, J.M. and E.P. Barton and J.A. Williams. 1979. Milk fat as related to vitamin B12status. J. Dairy Sci. 62: 642-645.
29. Erickson, P.S., A.M. Trusk and M.R. Murphy. 1990. Effects of niacin source on epinephrine stimulation of plasma nonesterified fatty acid and glucose concentrations, on diet digestibility and on rumen protozoal numbers in lactating dairy cows. J. Nutr. 120: 1648-1653.
30. Erickson, P.S., M.R. Murphy and J.H. Clark. 1992. Supplementation of dairy cow diets with calcium salts of long-chain fatty acids and nicotinic acid in early lactation. J. Dairy Sci. 75: 1078-1089.
31. Flachowski, G. 1993. Niacin in dairy and beef cattle nutrition. Arch. Anim. Nutr. 43: 195-213.
32. Frobish, R.A. and C.L. Davis. 1977. Theory involving propionate and vitamin B12 in the low-milk fat syndrome. J. Dairy Sci. 60: 268-273.
33. Fronk, T.J. and L.H. Schultz. 1979. Oral nicotinic acid as a treatment for ketosis. J. Dairy Sci. 62: 1804-1807.
34. Gee, J.M., A. Bhabuta and I.T. Johnson. 1989. A technique for assessing the biological availability of folate in foods. Food Chem. 31: 149-158.
35. Giles, W.H., S.J. Kittner, R.F. Anda, J.B. Croft and M.L. Casper. 1995. Serum folate and risk for ischemic stroke. First National Health and Nutrition Examination Survey Epidemiologic Follow-up study. Stroke. 26: 1166-1170.
36. Girard, C.L. and J.J. Matte. 1995. Serum clearance and urinary excretion of pteroylmonoglutamic acid in gestating and lactating dairy cows. Br. J. Nutr. 74: 857-865.
37. Girard, C.L. and J.J. Matte. 1996. Effects of dietary supplements of folic acid on lactational performance of dairy cows. J. Dairy Sci. 79 (Suppl. 1): 199.
38. Girard, C.L., J.J. Matte and G.F. Tremblay. 1989. Serum folates in gestating and lactating dairy cows. J. Dairy Sci. 72: 3240-3246.
39. Girard, C.L., J.J. Matte and J. Lévesque. 1992. Responses of serum folates of preruminant and ruminant calves to a dietary supplement of folic acid. J. Anim. Sci. 70: 2847-2851.
40. Girard, C.L., J. Chiquette and J.J. Matte. 1994. Concentrations of folates in ruminal content of steers: responses to a dietary supplement of folic acid in relation with the nature of the diet. J. Anim. Sci. 72: 1023-1028.
41. Girard, C.L., J.J. Matte and G.F. Tremblay. 1995. Gestation and lactation of dairy cows: A role for folic acid? J. Dairy Sci. 78: 404-411.
42. Glynn, S.A. and D. Albanes. 1994. Folate and cancer: a review of literature. Nutr. Cancer. 22: 101-119.
43. Grosse-Holz, D. and J. Harmeyer. 1988. Blut- und Milchparameter nach Nicotinsäurezulage bei laktierenden Kühen und Färsen. J. Anim. Physiol. a. anim. Nutr. 59: 182-191.
44. Halpin, C.G., D.J. Harris, I.W. Caple and D.S. Peterson. 1984. Contribution of cobalamin analogues to plasma vitamin B12 concentrations in cattle. Res. Vet. Sci. 37: 249-251.
45. Hargemeister, H. 1996. Effects of a long-term dietary biotin administration on claw health in dairy cows (a field study). Proc. 9th International Symposium on Disorders of Ruminant Digit. April 1996, Jerusalem.
46. Hayes, B.W., G.E. Mitchell, Jr., C.O. Little and N.W. Bradley. 1966. Concentrations of B-vitamins in ruminal fluid of steers fed different levels and physical forms of hay and grain. J. Anim. Sci. 25: 539-542.
47. Hoblet, K. 1996. Effect of biotin on claw health in first lactation Holstein cows. Proc. 9th International Symposium on Disorders of Ruminant Digit. April 1996, Jerusalem.
48. Hochstetter, T., Ch. Mülling and K.-D. Budras. 1996. Effects of biotin on the horn quality of the bovine claw. Proc. 9th International Symposium on Disorders of Ruminant Digit. April 1996, Jerusalem.
49. Horner, J.L., C.E. Coppock, G.T. Schelling, J.M. Labore and D.H. Nave. 1986. Influence of niacin and whole cottonseed on intake, milk yield and composition, and systemic responses of dairy cows. J. Dairy Sci. 69: 3087-3093.
50. Horner, J.L., C.E. Coppock, J.R. Moya, J.M. Labore and J.K. Lanham. 1988a. Effects of niacin and whole cottonseed on ruminal fermentation, protein degradability, and nutrient digestibility. J. Dairy Sci. 71: 1239-1247.
51. Horner, J.L., L.M. Windle, C.E. Coppock, J.M. Labore, J.K. Lanham and D. H. Nave. 1988b. Effects of whole cottonseed, niacin, and niacinamide on in vitro rumen fermentation and on lactating Holstein cow. J. Dairy Sci. 71: 3334-3344.
52. Hunkeler, A., Ch.-J. Lischer, H. Geyer and P. Ossent. 1996. The effect of biotin in the treatment of uncomplicated claw lesions with exposed corium in dairy cows. Proc. 9th International Symposium on Disorders of Ruminant Digit. April 1996, Jerusalem.
53. INRA. 1988. Alimentation des bovins, ovins et caprins. Ed. R. Jarrige. INRA. Paris, France.
54. Jaster, E.H. and N.E. Ward. 1990. Supplemental nicotinic acid or nicotinamide for lactating dairy cows. J. Dairy Sci. 73: 2880-2887.
55. Jaster, E.H., D.F. Bell and T.A. McPherson. 1983a. Nicotinic acid and serum metabolite concentrations of lactating dairy cows fed supplemental niacin. J. Dairy Sci. 66: 1039-1045.
56. Jaster, E.H., G.F. Hartnell and M.F. Hutjens. 1983b. Feeding supplemental niacin for milk production in six dairy herds. J. Dairy Sci. 66: 1046-1051.
57. Jennings, E. 1995. Folic acid as a cancer-preventing agent. Med. Hypotheses. 45: 297-303.
58. Johnson, R.R., O.G. Bentley, J.W. Hibbs and H.R. Conrad. 1956. In vivo and in vitronutritional requirements of rumen microorganisms. J. Agric. Food Chem. 4: 627-631.
59. Kennedy, D.G., P.B. Young, S. Kennedy, J.M. Scott, A.M. Molloy, D.G. Weir and J. Price. 1995. Cobalt - Vitamin B12 deficiency and the activity of methylmalonyl CoA mutase and methionine synthase in cattle. Internat. J. Vit. Nutr. Res. 65: 241-247.
60. Kon, S.K. and J.W.G. Porter. 1953. The B vitamin content of the rumen of steers given various diets. Proc. Nutr. Soc. 12: xii.
61. Kon, S.K. and J.W.G. Porter. 1954. Intestinal vitamin synthesis in ruminants. Vit. Horm. 12: 53-68.
62. Kung, L., Jr., K. Gubert and J.T. Huber. 1980. Supplemental niacin for lactating cows fed diets of natural protein or nonprotein nitrogen. J. Dairy Sci. 63: 2020-2025.
63. Lanham, J.K., C.E. Coppock, K.N. Brooks, D.L. Wilks and J.L. Horner. 1992. Effects of whole cottonseed or niacin or both on casein synthesis by lactating Holstein cows. J. Dairy Sci. 75: 184-192.
64. Lardinois, C.C., R.C. Mills, C.A. Elveiijem and E.B. Hart. 1944. Rumen synthesis of the vitamin B complex as influenced by ration composition. J. Dairy Sci. 27: 579-583.
65. Lee, L.A., J.D. Ferguson and D.T. Galligan. 1989. Effect of disease on days open assessed by survival analysis. J. Dairy Sci. 72: 1020-1026.
66. Loria, A., A. Vaz-Pinto, P. Arroyo, C. Ramirez-Mateos and L. Sanchez-Medal 1977. Nutritional anemia. VI. Fetal hepatic storage of metabolites in the second half of pregnancy. J. Pediatr. 91: 569-573.
67. Martinez, N., E.J. DePeters and D.L. Bath. 1991. Supplemental niacin and fat effects on milk composition of lactating Holstein cows. J. Dairy Sci. 74: 202-210.
68. McNulty, H., J.M. McPartlin, D.G. Weir and J.M. Scott. 1993. Folate catabolism is increased during pregnancy in rats. J. Nutr. 123: 1089-1093.
69. Metz, J. 1970. Folate deficiency conditioned by lactation. Am. J. Clin. Nutr. 23: 843-847.
70. Milligan, L.P., J.M. Asplund and A.R. Robblee. 1967. In vitro studies on the role of biotin in the metabolism of rumen microorganisms. Can. J. Anim. Sci. 47: 57- 64.
71. Minor, D.J., R.R. Grummer, R.D. Shaver and S.L. Trower. 1996. Effects of niacin and nonfiber carbohydrate on the metabolic status during the transition period and lactational performance. J. Dairy Sci. 79 (Suppl. 1): 199.
72. Morrison, H.I., D. Schaubel, M. Desmeules and D.T. Wigle. 1996. Serum folate and risk of fatal coronary heart disease. JAMA. 275: 1893-1896.
73. Muller, L.D., A.J. Heinrichs, J.B. Cooper and Y.H. Atkin. 1986. Supplemental niacin for lactating cows during summer feeding. J. Dairy Sci. 69: 1416-1420.
74. NRC. 1989. Nutrient requirements of dairy cattle. 6th Rev. Ed. National Academy Press. Washigton, USA.
75. Ottou, J.F. and M. Doreau. 1996. Influence of niacin on in vitro ruminal fermentation and microbial synthesis depending on dietary factors. Anim. Fedd Sci. Technol. 58: 187-199.
76. Potier de Courcy, G. and T. Terroine. 1979. Étude expérimentale du développement foetal dans la déficience en acide folique par antivitamines (acide X-méthyl-folique, méthotrexate). Ann. Biol. Anim. Biochim. Biophys. 19: 207-215.
77. Riddell, D.O., E.E. Bartley and A.D. Dayton. 1980. Effect of nicotinic acid on rumen fermentation in vitro and in vivo. J. Dairy Sci. 63: 1429-1436.
78. Riddell, D.O., E.E. Bartley and A.D. Dayton. 1981. Effect of nicotinic acid on microbial protein synthesis in vitro and on dairy cattle growth and milk production. J. Dairy Sci. 64: 782-791.
79. Roberge, I. and D. Gilbert. 1995. Annuaire statistique laitier du Québec, 1995, vol. 1. 9th Ed. Groupe de recherche en économie et politique agricole, Département d'économie rurale. GREPA, Université Laval, Québec.
80. Rulquin, H., P.M. Pisulewski, R. Vérité and J. Guinard. 1993. Milk production and composition as a function of postruminal lysine and methionine supply: a nutrient-response approach. Livest. Prod. Sci. 37: 69-90.
81. Rush, D. 1994. Periconceptional folate and neural tube defect. Am. J. Clin. Nutr. 59 (Suppl.): 511S-516S.
82. Schmid, M. 1995. Der Einfluss von Biotin auf die Klauenhornqualität bein Rind. Thesis. Veterinär-Anatomisches Institut des Universität Zürich.
83. Schwabb, C.G., C.K. Bozak, N.L. Whitehouse and M.M.A. Mesbah. 1992. Amino acid limitation and flow to duodenum at four stages of lactation. 1. Sequence of lysine and methionine limitation. J. Dairy Sci. 75: 3486-3502.
84. Schwabb, C.G., C.K. Bozak, N.L. Whitehouse and V.M. Olson. 1992. Amino acid limitation and flow to the duodenum at four stages of lactation. 2. Extent of lysine limitation. J. Dairy Sci. 75: 3503-3518.
85. Schussler, S.L., G.C. Fahey, Jr., J.B. Robinson, S.S. Masters, S.C. Loerch and J.W. Spears. 1978. The effect of supplemental niacin on in vitro cellulose digestion and protein synthesis. Internat. J. Vit. Nutr. Res. 48: 359-367.
86. Shields, D.R., D.M. Schaefer and T.W. Perry. 1983. Influence of niacin supplementation and nitrogen source on rumen microbial fermentation. J. Anim. Sci. 57: 1576-1583.
87. Skaar, T.C., R.R. Grummer, M.R. Dentine, R.H. Stauffacher. 1989. Seasonal effects of prepartum and postpartum fat and niacin feeding on lactation performance and lipid metabolism. J. Dairy Sci. 72: 2028-2038.
88. Treacher, R.J., I.M. Reid and C.J. Roberts. 1983. The effect of dietary niacin on the development of fatty liver in dairy cows. Proc. Fifth International Conference on Production Disease in Farm Animal. 166-169.
89. Tremblay, G.F., C.L. Girard, M. Bernier-Cardou and J.J. Matte. 1991. Nycterohemeral variations of concentration of serum folates in dairy cows. Can. J. Anim. Sci. 71: 919-923.
90. Walker, C.K. and J.M. Elliot. 1972. Lactational trends in vitamin B12 status on conventional and restricted-roughage rations. J. Dairy Sci. 55: 474-479.
91. Waterman, R. and L.H. Schultz. 1972a. Nicotinic acid treatment of bovine ketosis. II. Effects on long-chain fatty acid compositions of plasma lipid fractions. J. Dairy Sci. 55: 1454-1460.
92. Waterman, R. and L.H. Schultz. 1972b. Nicotinic acid loading of normal cows: Effects on blood metabolites and excretory forms. J. Dairy Sci. 55: 1511-1513.
93. Waterman, R., J.M. Schwalm and L.H. Schultz. 1972. Nicotinic acid treatment of bovine ketosis. I. Effects on circulatory metabolites and interrelationships. J. Dairy Sci. 55: 1447-1453.
94. Whitehead, V.M. and D.S. Rosenblatt. 1994. Folic acid supplementation and neural tube defects. Clin. Invest. Med. 73: 253-255.
95. Zimmerman, C.A., A.H. Rakes, T.E. Daniel and B.A. Hopkins. 1992. Influence of dietary protein and supplemental niacin on lactational performance of cows fed normal and low fiber diets. J. Dairy Sci. 75: 1965-1978.
96. Zinn, R.A., F.N. Owens, R.L. Stuart, J.R. Dunbar and B.B. Norman. 1987. B-vitamin supplementation of diets for feedlot calves. J. Anim. Sci. 65: 267-277.
Reference Books
1. Le Grusse, J. and B. Watier. 1993. Les vitamines. Données biochimiques, nutritionnelles et cliniques. Centre d'Étude et d'Information sur les Vitamines. Produits Roche, Neuilly-sur-Seine, France.
2. McDowell, L. R. 1989. Vitamins in Animal Nutrition. Comparative Aspects to Human Nutrition. Academic Press, Inc., San Diego, CA.
3. Weil, J.-H. 1972. Biochimie générale. Masson et Cie, Paris, France.