Department of Animal Science, University of Tennessee,
Knoxville, TN 37901-1071, U.S.A.
E-mail: jquigley@utk.edu
Take Home Message
Raising calves from birth to weaning requires considerable management and attention to detail. Four critical areas are most important to successful calf raising: colostrum management, liquid feeding, calf starter and ruminal development, and housing. Colostrum quality, quantity fed, and timing of colostrum feeding determine the acquisition of passive immunity and resistance to disease prior to weaning. Liquid feeding (milk, milk replacer, or alternative liquid sources) provides nutrients for maintenance and limited body weight gain prior to weaning. Calf starter and water are important to initiating the development of ruminal function and will affect the age at which calves may be weaned. Housing is important to minimize exposure to pathogens and provide a comfortable, stable environment for the animal. When properly managed, calves can often be weaned at four weeks of age.
Introduction
The replacement enterprise is a pivotal component of most modern dairy farms. By providing a consistent and economical supply of high quality replacements to the lactating herd (or for sale to other herds), the replacement enterprise can be viewed as a profit (or loss) center for the dairy. This enterprise approach to calf rearing, which requires sound business and management decisions, can allow producers to evaluate the replacement enterprise and identify areas where the enterprise may be improved.
Calves are born with a predetermined genetic potential, which may be permanently affected by management decisions implemented throughout the rearing period and by environmental factors. A calf's genetic potential may be viewed as an upper limit that is expressed only if proper decisions are implemented at the appropriate time. Studies have shown that the level of management has a profound effect on calf morbidity and mortality (7, 14, 15, 38, 39). Proper management of young stock, particularly during the neonatal period, can markedly reduce morbidity and mortality, whereas improper management will lead to economic losses from increased cost of veterinary intervention, death losses, reduced growth, and suboptimal reproductive performance. In addition, poor management of young stock can reduce the lifetime productivity of the individual cow and the herd as a whole.
The most critical time in the life of the dairy replacement is during the first few days, when morbidity and mortality are greatest. A recent USDA study of farms throughout the U.S. with more than 30 cows (25) indicated that preweaning mortality of calves born alive was 8.4%, whereas mortality after weaning was only 2.2%. Clearly, the loss of calves prior to weaning is a major concern for all dairy producers.
Dry Cow Management and Calving
Health and profitability of the calf begins prior to birth. Because the calf is especially susceptible to pathogens during the first few hours after birth, the calving environment is critical. In addition, preparing the dry cow for calving by proper nutrition and management will contribute significantly to the health of the calf after calving.
Use of a Hygienic Calving Area and Assistance
A hygienic environment at the time of birth is important for the health of the calf. Feet, hindquarters, perineum, and udder of the dam should be cleaned before parturition. When possible, maternity pens should follow the all-in-all-out system with disinfection in between to prevent transition of pathogens. Ideally, cows should calve in pasture that is clean and dry and that preferably has shade. The NAHMS study (25) found that pasture resulted in low calf mortality. Obviously, the pasture must have good drainage to minimize mud and allow the cow to isolate herself for calving.
The incidence of dystocia should be <15% in heifers and <8% in multiparous cows. The percentage of dystocia is dependent on the gender of the calf, breed, bull choice, position at the time of birth, body condition of the dam, and parity of the dam. Any calf born as a result of dystocia is much more prone to stillbirth, neonatal mortality, and colostrum deprivation (38, 39). The chance for neonatal acidosis (metabolic and/or respiratory) are markedly increased when calves experience a difficult birth.
Navel Disinfection
The navel cord should be disinfected shortly after birth (12). This helps dry the cord and keeps organisms from entering the body. Studies have shown that calf morbidity (particularly enteritic and respiratory diseases) and mortality are reduced when the cord is dipped shortly after birth. A 5% iodine solution should be used, and not dilute iodine solutions such as teat dip. Alcohol included in tincture of iodine can further reduce the risk of infection and speed drying of the cord. The NAHMS study (25) revealed that only about 47% of calves have their cords dipped. The stump should be rechecked for infection during the first three days after birth. If the umbilical cord should break just outside the abdominal wall, the naval should be stitched immediately. This is frequently seen when calves are born in a posterior position and in combination with a cesarean section.
Stimulating the calf may be done by the dam or by the producer. If the calf is removed from the dam immediately after birth, it should be rubbed with straw or a clean dry towel to dry the calf. This will also stimulate breathing and circulation and help the calf to stand early. When the weather is cold, a heat lamp will help to keep the calf warm while it dries off after birth.
Colostrum Feeding - Birth To Day Four
Introduction
Regardless of the method of feeding colostrum, a key component to a successful calf management program is an adequate concentration of serum immunoglobulin G (IgG). Because serum IgG concentration is so closely related to the calf's overall resistance to infectious agents (e.g., viruses, bacteria, parasites), it has been used as a measure of overall protection provided by the dam. Although the level of IgG that provides adequate protection will vary with the particular situation (pathogen load in the environment, stress, housing, feeding, etc.), a level of 10 g/L has been suggested as a reasonable goal for IgG in the serum of calves by about 24 hours of age. The condition of serum IgG 10 g/L is termed failure of passive transfer (FPT) and is associated with increased morbidity and mortality.
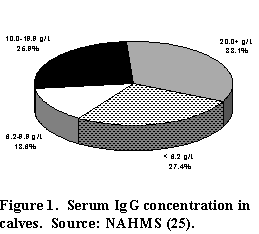
In the NAHMS study of U.S. dairy herds (25), 2,177 heifer calves (from 593 farms) were used to assess serum immunoglobulin (Ig) concentrations. Blood from these calves was sampled between 24 and 48 hours of age and serum was measured for IgG, which comprises most of the Ig in blood. Serum IgG is responsible for most of the calf's resistance to disease and is obtained by consumption of colostrum during the first 24 hours after birth.
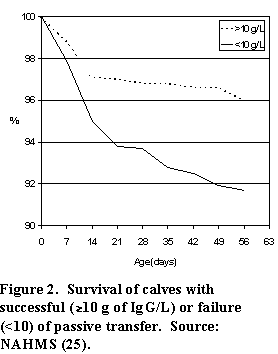
Over 40% of all calves sampled had IgG concentrations below the recommended level of 10 g/L (Figure 1). Worse still, over 25% of calves were below 6.2 g/L, which put them at a much greater risk of disease than calves with higher IgG levels. Mortality rates of calves with serum IgG less than 10 g/L was over twice that of calves with higher IgG levels (Figure 2). Of course, many other factors contribute to calfhood mortality, but this study indicated that over half of the death loss of calves with serum IgG levels less than 10 g/L (about 40% of dairy heifer calves) can be attributed to lack of IgG intake.

For many years researchers, extension specialists, and dairy professionals have recommended early feeding of a large amount of colostrum to provide Ig prior to closure of the small intestine. Recent research has indicated the continued importance of feeding colostrum and has further refined our understanding of the importance of colostrum to the young calf. Not only does colostrum provide vital Ig, but it also provides significant amounts of immune proteins and nutrients that support the calf during the first few days of life.
Absorption of Ig
Absorption of intact macromolecules across the intestinal epithelium into the neonatal circulation is possible for approximately 24 hours after the calf is born. The absorption of Ig occurs by an active process called pinocytosis, which moves Ig (and other molecules) across the intestinal epithelium. After leaving the epithelium, Ig molecules move into the lymph and then to the circulation. Maturation of the small intestine begins shortly after birth and the ability of the intestine to absorb macromolecules without digestion is lost by about 24 hours after birth. This loss of absorptive ability (also called closure) appears related to the development of the digestive apparatus in intestinal epithelial cells and turnover of cell populations. After about 24 hours of age, the chance to provide the calf with antibodies is gone. However, it is important to continue to feed colostrum for two to three days after birth. The Ig in colostrum will bathe the calf's digestive tract and make it difficult for bacteria to attach to the intestinal wall. This "local effect" can reduce the incidence of scours during the first several weeks of life.
Antibodies have different fates in the animal. IgA is primarily responsible for inhibiting the attachment of pathogens in the intestinal tract; therefore, the role it plays is mainly associated with mucosal surfaces (including the intestine). Immunoglobulin M is a large molecule that generally stays in the circulation. It is especially important as a primary defense against bacterial infections. On the other hand, IgG equilibrates with several body pools, so blood IgG concentration reflects the concentration in only one of those pools. However, there is a strong relationship between serum IgG concentration and morbidity and mortality, so serum IgG concentration has been used extensively to determine success or failure of passive transfer from the dam to the neonate.
Specificity of Ig is Critical to Disease Resistance
The relationship between serum IgG concentration and calf health is highly significant in most studies. However, the calf's susceptibility to disease is a response not only to the degree of protection provided by cellular and humoral immunity, but also the exposure to pathogens in the environment and the animal's physiological state. Calves with a full complement of antibodies may develop disease if the number of pathogens in the environment is overwhelming or if the pathogen challenge is one to which the calf has no immunity. Because Ig are specific for specific antigens, the calf must obtain a wide array of different Ig to provide protection. If the Ig in colostrum are not those specific for antigens present on the farm, then the Ig concentration in the blood (however high) will provide no immunity. This may be the case when calves are housed in facilities physically removed from the older cows, or when colostrum supplements are used. Finally, animals that are stressed due to climate (extreme heat or cold), poor housing and ventilation, or inadequate nutrition may be at a greater risk of developing disease.
Balance between Immunity and Pathogen Load
In normal, healthy animals, the immune system acquires a balance, or control, over the pathogens to which the body is exposed. In the neonate, this balance is profoundly dependent upon the passive immunity received by the calf from the dam during the first 24 hours of life. It is important to note that even high levels of serum IgG cannot always prevent development of enteric, respiratory, or other disease. When the number of pathogens in the environment overwhelms the ability of the immune system, however competent, the result will be development of disease. Thus, a critical component of calf management is proper environmental (or pathogen) management. Reducing the number and types of pathogens to which the calf is exposed can relieve the immune system from constant attack, thereby allowing the calf to expend energy on more productive functions, such as maintenance and body weight gain. Many dairy producers can markedly improve calf survival by reducing the challenge to which their calves are exposed without making significant changes to their current colostrum feeding practices.
Methods of Feeding Colostrum and Quantity of Colostrum
Allowing the Calf to Nurse
Allowing the calf to nurse the dam for one or more days has been shown to be an inadequate method of supplying colostrum in many research studies. Although many producers (>30%; 25) allow their calves to suckle the dam, a considerable body of research indicates that many calves, up to 40%, may obtain insufficient Ig with this method of feeding. When calves suckle the dam, it is very difficult to know when the calf begins to nurse and how much colostrum it consumes. Calves having difficult births, and calves from heifers are at particular risk of FPT due to insufficient colostral consumption. Further, calves often have a difficult time finding the teats of cows with large, pendulous udders, and may consume inadequate colostrum. Because of the high degree of FPT when calves are left to suckle the dam, most dairy professionals recommend separating calves from the dam and feeding colostrum by a nipple-bottle or esophageal feeder (if the calf will not consume enough colostrum by bottle).
Bottle Feeding Colostrum
Feeding calves by nipple bottles has two inherent advantages: it allows the producer to control the time at which calves are fed and allows the producer to control (at least partially) the amount fed to the calf. Feeding by nipple is the preferred method of feeding colostrum to calves. Most producers (64%) use nipple bottles to feed colostrum to calves in the first 24 hours after birth.
The amount of colostrum to be fed by nipple bottle should depend on size of the calf, age at first feeding, quality of colostrum, and other factors. However, the mass of IgG in colostrum is the most important factor affecting successful transfer of immunity in calves (5). Traditionally, feeding of two liters (quarts) of colostrum at two feedings has been recommended. However, due to concerns about poor colostrum quality (low IgG concentration), many researchers and veterinarians now recommend four liters (quarts) at the first feeding (4). A recent producer guide (3) suggested that if colostrum quality is estimated with a colostrometer, two liters of good quality colostrum can be fed in each of two feedings; if quality is not determined, then three liters of colostrum should be fed in the first feeding. Calves will generally consume three or more liters by nipple bottle when they are healthy and stand within about an hour of birth. However, if the producer desires to provide four liters (one gallon) of colostrum to calves at the first feeding, an esophageal feeder may be necessary.
Esophageal Feeder to Feed Colostrum
Use of an esophageal feeder is becoming an increasingly popular method of providing colostrum to calves. The esophageal feeder has several inherent advantages, including control of the time of colostrum feeding control of the amount of colostrum fed, and the ability to force the calf to consume a known (usually large) amount of colostrum at the first feeding. Disadvantages of using the esophageal feeder include delivering the colostrum to the rumen and possibility of delivering colostrum into the lungs. About 2% of producers were regularly feeding colostrum by esophageal feeder in the U.S. in 1992 (25). However, many veterinarians are recommending this practice, so the application is likely to become more prevalent.
Use of the esophageal feeder to feed large quantities of colostrum has been associated with lower apparent efficiency of IgG absorption (AEA) and slightly lower serum IgG concentration compared to feeding colostrum by nipple bottle (18). When colostrum is administered by esophageal feeder, the colostrum enters the rumen before moving into the abomasum and intestine (17). It takes two to four hours for the colostrum to leave the rumen. This may actually be the reason for lower AEA, as the intestine matures during this time, reducing the number of actively absorbing cells in the intestine. However, many veterinarians recommend feeding four liters of colostrum as soon as possible after birth, to ensure that all four liters are consumed. Others (2, 23) support the use of esophageal feeders to provide large amounts of colostrum without significant effect on serum IgG concentrations.
Use of the esophageal feeder is most useful when colostrum quality cannot be determined. Because it is not possible to determine with great precision the quality of colostrum, there is a risk that an insufficient mass of Ig will be provided to the calf in the first feeding. Additionally, the efficiency with which Ig are absorbed declines as the calf ages, so the first feeding may be the most important. Thus, the recommendation has been made to use a large first feeding (four liters) and not be concerned about significant consumption at a second feeding 12 hours later.
Colostrum Quality
The amount of IgG absorbed depends on the calf's ability to absorb IgG (efficiency of absorption) and the mass of IgG consumed. The mass of IgG consumed is a function of the quantity of IgG x the IgG concentration of the colostrum. The concentration of Ig in colostrum varies according to the cow's disease history, volume of colostrum produced, season of the year, breed, and other factors. Research from Washington (26) indicated the average concentration of IgG1 (a sub-fraction of IgG) in colostrum from 919 Holstein cows was 48.2 g/L with a range of 20 to >100 g/L. A Tennessee study (32) measured colostrum from 96 Jersey cows and found that samples averaged 66 g/L of IgG, with a range of 28 to 115 g/L. The difference between 20 and 100 g/L of IgG in colostrum can mean the difference between FPT and successful passive transfer.
The amount of Ig in colostrum depends on a large number of factors, including the disease history of the cow. That is, cows tend to produce Ig in response to pathogens to which they have been exposed. Therefore, cows exposed to a greater number of pathogens tend to produce colostrum with greater Ig than cows exposed to fewer pathogens. This is often why older cows will produce colostrum containing more Ig than younger cows. However, if older cows are not exposed to many pathogens, the colostrum produced may not have high levels of Ig. This is also why a good dry cow vaccination program can improve the quality of colostrum. Moreover, cattle raised on a farm will produce colostrum with antibodies specific for the organisms on that farm, which is an added benefit. Finally, prepartum milking or leaking of milk from the udder prior to calving can reduce the concentration of Ig in colostrum.
Research has also indicated that the volume of colostrum produced will influence colostral Ig concentration. In general, colostrum produced in large volumes will have lower Ig concentration than colostrum produced in smaller volumes. This is only a general rule, however, and the relationship between Ig concentration and volume is not constant.
The large variation in Ig content makes accurate colostrum management and feeding difficult. Colostral IgG can be measured in the laboratory with great accuracy; unfortunately, the assays involved are time-consuming and expensive. A measurement of colostrum specific gravity using a device called a colostrometer is one method to estimate Ig content of colostrum (9). This device is based on the relationship between Ig in colostrum and specific gravity. Unfortunately, components of colostrum other than Ig affect specific gravity, so the relationship is variable. Also, the relationship between specific gravity and IgG is dependent on temperature (21, 22, 27). However, the colostrometer may give a gross (qualitative) estimate of colostrum quality, particularly if the colostrum is of poor quality.
Colostral Supplements
Maternal colostrum is almost always preferable to a colostrum supplement due to the mass of Ig available and the nature of the IgG in colostrum. The IgG in maternal colostrum are derived from the dam's blood stream and are based on the disease history to which the cow has been exposed. Conversely, IgG from colostral supplements are derived from pooled colostrum from many cows. These cows may not have been exposed to diseases that are typical for a farm, and therefore, calves may not receive the appropriate type of immunity. Use of colostral supplements has been evaluated at several locations. This research has indicated relatively poor absorption of IgG from colostral supplements. Abel and Quigley (1) added a colostral supplement to maternal colostrum from 32 cows. Colostrum ranged from very high to low concentration of IgG, and averaged 59 g of IgG/liter. Calves were fed colostrum by 2 hours of birth, and again 12 hours later. No effect of colostral supplement was observed on serum IgG concentrations taken 24 or 48 hours after birth (Figure 3). These data indicate that when maternal colostrum was fed, there was no benefit to adding a colostrum supplement. When only the poor quality colostrum samples (<20 g of IgG/L) were evaluated, the results were similar. Even when poor quality colostrum was fed, there was little benefit to adding a colostral supplement.
In a second study, use of colostral supplements was reevaluated by feeding newborn calves two liters (quarts) of dam's colostrum as soon as possible after birth and again 12 hours later. This study reconfirmed our previous finding that colostrum supplements do not affect serum IgG concentrations when added to good quality colostrum. Generally, the use of the current generation of colostrum supplements has shown poor absorption and only limited increases in serum IgG concentrations when fed at or above manufacturer's recommendations (11 ).
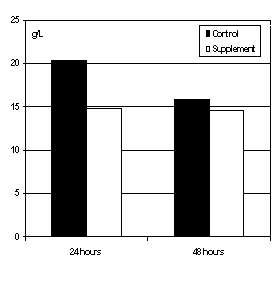 Figure 3. Concentrations of serum IgG in
Holstein calves at 24 and 48 hours when fed
maternal colostrum with or without colostral
supplement (1).
Figure 3. Concentrations of serum IgG in
Holstein calves at 24 and 48 hours when fed
maternal colostrum with or without colostral
supplement (1).
Timing of Colostrum Feeding
The time at which colostrum is first fed has a critical role in determining if the calf will acquire adequate passive immunity and whether it becomes ill. The timing of colostrum feeding is important for two reasons: loss of absorptive sites and bacterial colonization of the intestine.
Loss of Absorptive Sites in the Intestine
As mentioned above, maturation of the intestine begins shortly after birth. Current theories suggest that intestinal epithelial cells lose their ability to absorb intact macromolecules after about 24 hours due to maturation of the cells and development of the cellular digestive apparatus; however, this maturation begins shortly after birth. Rajala and Castrén (35) reported a decline in serum IgG concentration of 2 g/L at 30 min after birth; regression of serum IgG concentration on age at first feeding in calves fed maternal colostrum (1) also indicated a reduction of AEA within 1 hour of birth. Clearly, there is a compelling reason to feed calves as soon as possible after birth to maximize acquisition of passive immunity.
In addition to maturation of intestinal cells, the secretion of digestive enzymes in the abomasum and intestine of the calf may also contribute to lower AEA by degrading IgG prior to absorption. At birth, and for a limited period thereafter, the secretion of digestive enzymes remains limited to allow macromolecules such as IgG to escape digestion and allow absorption (36). However, by about 12 hours, enzyme secretion becomes more marked, thereby reducing the ability of IgG molecules to reach the peripheral circulation.
Bacterial Colonization of the Intestine
The intestinal tract of the neonate is sterile at birth; however, within a few hours bacteria from the environment begin to colonize the intestine. This colonization can be hastened by an environment that promotes the growth of pathogens (i.e., a dirty environment). If a calf is born into an environment containing large numbers of pathogenic bacteria, the chances of colonization by pathogenic bacteria is increased. This may lead to septicemia, leading to severe morbidity and, often, mortality. Further, James et al. (13) reported that the presence of bacteria in the intestine may actually speed closure, thereby reducing the acquisition of passive immunity.
Logan et al. (20) studied the effects of early colonization of pathogens on neonatal calves. Calves were fed colostrum and challenged with E. coli. The first group was fed colostrum, then challenged; group 2 were challenged, then fed colostrum. Nearly all calves in the second group became morbid, and about 75% of the group died. Conversely, calves fed colostrum prior to E. coli challenge did not become sick and none died.
Quigley et al. (31) reported that calves removed from their dams at birth showed different temporal acquisition of enteric pathogens from birth to 35 days compared to those left with the dam for 24 hours. Clearly, the dam and the calving environment can contribute significantly to the amount and type of bacteria to which the calf is exposed shortly after birth.
Storing Colostrum
Storage can also influence colostrum quality. Colostrum can be refrigerated for only about one week before quality (Ig concentration) declines. For long-term colostrum storage, freezing is the best alternative. Colostrum may be frozen for up to a year without significant decomposition of Ig, and one report indicated that colostrum has been stored for up to 15 years without serious deterioration. It should be noted that frost-free freezers are not optimal for long-term colostrum storage, as they go through freeze-thaw cycles that allow the colostrum to thaw and refreeze. This can markedly shorten colostrum storage life.
Freezing colostrum in 1 or 2 liter bottles or 1 quart in 1 or 2 gallon zip-closure storage bags is an excellent method of storing colostrum. When needed, these bottles or bags can be placed in warm (not hot) water and allowed to thaw. Alternately, colostrum can be thawed in a microwave oven with little damage to the Ig. It is important to microwave the colostrum for short periods on low power and pour off the thawed liquid periodically to minimize heating. It is also important to avoid "hot spots" inside the frozen colostrum. Use of a turntable can help to minimize damage to Ig.
Liquid Feeds for Preweaned Calves
Digestion of Liquid Feeds by Young Calves
Prior to rumen development, the functional compartment of the calf's stomach is the abomasum. Milk, colostrum, or milk replacer feeding will bypass the rumen by closure of the esophageal (reticular) groove, causing the liquid to travel from the esophagus to the omasum and abomasum. Closure of the reticular groove occurs by a neural stimulation in response to feeding. Note that closure will occur when calves are fed from buckets or bottles, and whether the liquid is warm or cold. Also, water will not cause closure of the reticular groove, and enters the rumen when consumed.
Milk or milk replacer consumed by-passes the rumen; therefore, digestion of these liquids will occur in the abomasum (true stomach) and intestine. The site of digestion depends somewhat on the type of liquid. Whole and waste milk and colostrum will form a clot caused by the production of rennin in the abomasum. This acts to hold fat and casein in the abomasum, thereby slowing flow into the intestine. Most modern milk replacers contain little casein, so a clot will not form in the abomasum when milk replacers are fed. In most cases, this does not markedly affect digestibility or metabolizability of energy or protein.
Nutrient digestibility increases with age to about three weeks of age due to maturation of tissues secreting digestive enzymes. Calves 3 weeks of age are especially susceptible to lower quality ingredients in milk replacer, although milk and milk ingredients in milk replacers are digested well.
Sources of liquids for calves prior to weaning include whole milk, waste milk, excess colostrum, and milk replacer. Most U.S. dairy producers use some combination of all of these sources.
Whole milk is an excellent feed for calves prior to weaning, but is expensive compared to other sources of liquid feed. In most cases, whole milk is the most expensive source of liquid and, thus, is not the best liquid source for calves prior to weaning. However, whole milk contains more protein and energy than most milk replacers (Table 1), and is second only to excess colostrum in nutrient content. Anecdotal reports indicate that some producers have success in raising calves only when whole milk is used; this has been attributed to factor(s) inherent in the milk, such as Ig, protein, or other components. However, this may be more a function of level of management than some component of whole milk. The amount of whole milk to feed is approximately 10% of body weight, with consideration given to health of the calf and environmental conditions. Excessive milk feeding can lead to nutritional scours and delayed consumption of calf starter.
Table 1. Comparison of whole milk and selected milk replacers1.
| Item | Whole milk | 20:20
All milk |
22:15 SPC | 22:20
All milk |
| DM, % | 12.5 | 95.0 | 95.0 | 95.0 |
| Protein, % of DM | 26.4 | 21.1 | 23.2 | 23.2 |
| Fat, % of DM | 28.0 | 21.0 | 15.8 | 21.1 |
| Crude fiber, % of DM | 0.0 | 0.2 | 0.2 | 0.2 |
| ME, Mcal/kg | 4.9 | 4.5 | 4.2 | 4.5 |
| Ca | 0.95 | 0.8 | 0.8 | 0.8 |
1Composition of individual milk replacers may vary from data contained herein due to differences in ingredients and processing.
Milk replacers are an excellent option for feeding liquid to calves prior to weaning. Milk replacers used in the U.S. are composed of whey and whey protein, fats, and minerals and vitamins (Table 2). Some replacers utilize vegetable protein (soy, wheat, potato) or animal protein (fish, plasma, erythrocyte) to replace some or all of the milk protein from whey protein concentrate. Due to its high price, little skim milk is used in modern milk replacers. Milk replacers have been used successfully by many producers for many years and they are the feed of choice for most feeders of preweaned replacement as well as veal calves.
Composition of milk replacers will influence the performance of calves prior to weaning. Important factors include source and amount of protein and energy, vitamin and mineral supplementation, and inclusion of critical nutritional additives such as emulsifiers. Unfortunately, methods traditionally used to determine milk replacer quality are not useful with modern replacers used in the industry. For example, rennet coagulation was used as a method of determining the amount of casein in a milk replacer, and thus its "quality". However, few (if any) modern milk replacers contain casein (a component of skim milk), so none will form a rennet clot. This does not mean that all replacers are poor quality. Whey proteins can support growth as well as casein protein. Another method of determining protein quality is crude fiber content. The crude fiber content of a milk replacer will indicate the relative amount of vegetable protein added to a milk replacer; it will not indicate whether that protein is digestible. Milk replacers can contain soy proteins that have been chemically modified to increase their digestibility and reduce the antigenicity of proteins such as glycinin and -conglycinin and denature trypsin inhibitors. These milk replacers perform quite acceptably, and are usually available at a less expensive price compared to all-milk replacers. Furthermore, some vegetable protein sources (e.g., soy isolate) contain no crude fiber, and thus, measuring crude fiber will not necessarily indicate the presence of vegetable protein.
Table 2. Typical ingredient and nutrient composition of milk replacers1.
| Ingredient, % | All-milk | Soy isolate | Soy protein concentrate | Soy flour | Animal plasma |
| Whey protein concentrate | 44.5 | 7.0 | 9.2 | 41.4 | |
| Delactosed whey | 10.0 | 10.0 | 10.0 | 8.5 | |
| Dried whey | 25.2 | 50.8 | 19.8 | 46.5 | 28.2 |
| Soy isolate | 11.2 | ||||
| Soy protein concentrate | 15.0 | ||||
| Soy flour | 33.8 | ||||
| Animal plasma | 7.5 | ||||
| Fat | 19 | 19.5 | 14.5 | 9.7 | 20.0 |
| Mineral/vitamin/amino acids | 1.3 | 1.5 | 1.5 | 1.5 | 2.8 |
| Nutrient, % | |||||
| Protein | 20.0 | 20.0 | 21.0 | 24.0 | 20.0 |
| Fat | 20.0 | 20.0 | 15.0 | 10.0 | 20.0 |
| Fiber | 0.15 | 0.15 | 0.5 | 1.0 | 0.15 |
1 Composition of individual replacers may vary.
Adapted from: Tomkins & Jaster (37); Quigley and Bernard (34).
Determining milk replacer quality is best determined by animal performance. However, some factors that are related to performance include: 1) a reputable manufacturer; 2) analysis of replacer (protein and fat); 3) ingredients used; 4) level of medication; and 5) other characteristics (24). Milk replacers should not contain off-color materials, should mix quickly and evenly, and stay in solution.
Method of preparation and feeding can influence performance of calves fed commercial milk replacers. Proper emulsification requires sufficient temperature - use warm, not cold water!
Excess colostrum is an excellent feed for young calves. It is high in DM and protein, and low in lactose. It may be stored refrigerated in small bulk tanks or refrigerators for several weeks. Due to its higher DM content, surplus colostrum may cause calves' feces to become somewhat "loose". However, this is not usually a significant problem, and can be alleviated by diluting the colostrum (3:1 or 4:1) with water. Recent research suggests that antibodies in surplus colostrum may contribute somewhat to intestinal immunity (8, 10), however, large-scale studies using surplus colostrum have not been completed.
Waste milk is that milk that is unsaleable due to mastitis or treatment with antibiotics. It is often considered a "free" source of liquid to feed to their calves. However, the opportunity cost of waste milk is significant (i.e., the value of that milk if it weren't waste). In most cases, it is more economical to reduce the production through management and/or culling and feed an alternative source of liquid. Several precautions should be followed when using waste milk: 1) do not use milk from the first milking after antibiotic treatment because this milk contains too much antibiotic and may lead to residue problems; 2) do not use milk that is excessively bloody or unusual in appearance; 3) do not feed waste milk to group housed calves; and 4) do not use milk from cows infected with Escherichia coli or Pasteurella. Finally, do not feed waste milk to calves held for short periods (e.g., bull calves for sale). Antibiotics in waste milk can carry over in the animal's tissues resulting in significant antibiotic residues.
The amount of liquid (milk, colostrum, milk replacer) has often been associated with the amount of growth attained by calves prior to weaning. A closer evaluation of the amounts of energy and protein provided by liquids under current feeding programs indicates that this assumption is incorrect. Under most situations, milk or milk replacer feeding provides sufficient energy to support only a limited amount of body weight gain. Although the amount of gain supported by liquids depends on many factors (body weight of calf, climatic conditions, health of the calf, etc.), it will generally support less than 400 grams of body weight gain per day, and usually significantly less than 400 grams/day. Most body weight gain will be obtained by consumption of calf starter, not liquid feeds.
Effects of Environment on Liquid Feeding
Because calves are fed for limited body weight gain from liquid feeds, the importance of the calf's environment becomes especially important. An amount of milk replacer that will support 250 grams of body weight gain in thermoneutral conditions will not support the same gain when the temperature is -30°C. Generally, when the temperature is below freezing, additional energy should be supplied. When feeding milk replacer, this can be achieved by increasing the amount of fat (e.g., increasing from 10% fat to 20% fat) or by increasing the amount of powder added to each bottle or bucket. When milk or colostrum is fed, added amounts are necessary. In addition, commercial fat supplements may be added to milk or milk replacer to increase the energy density.
Feeding equipment usually consist of nipple bottles, buckets, and nipple pails. Calves readily adapt to nipple bottles, and training calves to drink is much easier. Training calves to drink from a bucket is initially more difficult, but buckets are easier to clean, and research indicates that they generally result in lower mortality. Nipple buckets are most difficult to clean, and thus are a choice only when good management is involved. Calves fed from buckets (or from a nipple with an enlarged opening) will experience some spillage of liquid into the rumen. Sanitation of all equipment is critical to the health and growth of calves. Hot water and disinfecting solution are important to inhibit the growth of bacteria (milk is an excellent growth medium for bacteria) and reduce the spread of disease.
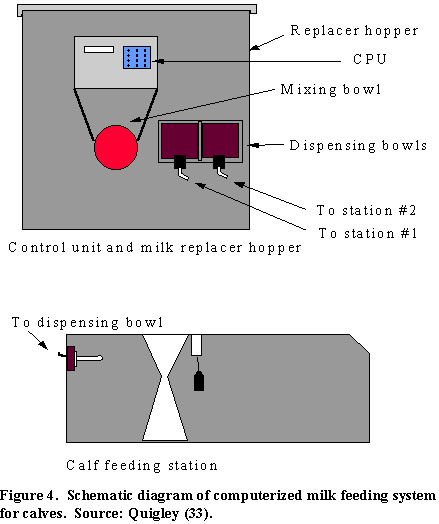
Recent developments in feeding methods include use of computerized milk replacer feeding systems (33) and group ("mob") feeders. Computer milk replacer feeding systems have been used in Europe for a number of years, and have been used on some farms in the U.S. over the past few years. Basically, the computer system is similar to the ones used to feed grain to lactating dairy cows (Figure 4). Each calf is fitted with a transponder that uniquely identifies it to the computer. When the calf enters the feeding station, it is identified by the computer that controls the system. Each calf is allotted a fixed amount of milk or milk replacer in a 24-hour period. This is usually divided into eight meals, each of which can occur every three hours. If a calf hasn't eaten in the past three hours, it is allowed to eat. When allowed to eat, the computer will measure a predetermined amount of milk replacer powder into a mixing bowl. Warm water is added to the proper concentration (usually to make a 500 ml meal) and mixed thoroughly. The reconstituted milk replacer is then transferred to a feeding bowl, which is connected to the heavy duty nipple. Calves then drink the reconstituted milk replacer. If a calf enters the station before it is allowed another meal, the animal is allowed to nurse on the nipple, but no milk replacer is delivered. Usually calves are allotted eight 500 ml meals per day to make 4 liters. Evaluations of such systems indicate that they can be used successfully in many types of calf rearing programs; they are especially useful when labor is particularly expensive.
Mob feeders are a simpler approach to group feeding. A large barrel is fitted with several (5 or more) heavy duty nipples. Calves may be limit-fed milk or replacer from the mob feeder, or liquid may be available for ad libitum consumption. Calves fed ad libitum will consume markedly more milk replacer, thereby increasing costs. In some cases, milk replacer is acidified to reduce intake.
Weaning
When calves are weaned, the cost of rearing declines considerably. Whole milk or milk replacer is markedly more expensive than calf starter and hay, and labor associated with feeding liquids is reduced also. Thus, it makes economic sense to wean calves as soon as is reasonable. Traditionally, many researchers and extension professionals have recommended that calves be weaned at four weeks of age. It was argued that at this age, calves have sufficient ruminal development to allow them to obtain adequate nutrients from calf starter alone. However, according to the NAHMS (25) study, only about 10% of producers wean calves at 4 weeks of age, indicating that producers have discounted this recommendation for their own reasons. An informal survey of veterinarians also raises question about early weaning; many veterinarians recommend weaning at eight weeks or later.
There are other economic benefits to promoting ruminal development in addition to reduced feed costs. After weaning, calves are less susceptible to disease and gain more body weight with lower labor and management costs. Therefore, it is usually most economical to manage calves to promote early rumen development and wean calves as early as is feasible.
Criteria for Weaning
According to the NAHMS (25) study of dairy calf management practices, most dairy producers use age of the calf as the primary criterion for weaning. The most common age at weaning is 8 weeks (32.9% of producers), although a few (2.3%) wean at 3 weeks of age and others (21.4%) wean at 10 weeks of age or later. Some producers wean calves when calves starter intake reaches a predetermined level (usually 700 to 1,000 grams/day) or when calves reach a preselected body weight.
Methods of weaning include abrupt or gradual weaning. Abrupt weaning is accomplished by simply ending milk feeding according to a specific criterion (e.g., intake, age, etc.). Gradual weaning occurs when the amount of liquid offered is reduced for a period of time prior to complete removal of liquid. Often, the amount is reduced by feeding only once daily instead of the normal twice-daily feeding for five to seven days prior to complete removal of liquid. This is thought to stimulate dry feed intake while still allowing the calf to obtain nutrients from liquid and, possibly, reduce stress associated with abrupt weaning. In general, if the rumen has developed sufficiently prior to removal (or reduction) of liquid feeding, either method is satisfactory. Gradual weaning often results in unhappy (i.e., noisy) calves, particularly at the feeding time when they are not fed.
A significant problem associated with weaning according to age is the assumption that calves will consume a sufficient amount of dry feed to stimulate rumen development by a certain age. It is important to note that preparation for weaning is a function of dry feed intake, not age (see below). Although this relationship between age and intake may be acceptable in most cases, individual calves might not have consumed sufficient feed to allow them to obtain sufficient nutrients from feed after weaning.
Weaning stress is caused by the reduction in energy intake associated with weaning. An example of this decline in energy intake might be useful. At 6 weeks of age, a calf may be consuming 700 grams of calf starter and 450 grams of milk replacer dry matter. At weaning, the calf will receive only 700 ÷ 1150 = 61% of its previous intake. This deficit in energy and protein will cause the animal to go into negative energy balance if its starter intake does not increase to make up the difference. In addition, liquid feeding is an inherently pleasurable experience for the animal, and weaning will be associated with stress caused by terminating that experience.
Stress at weaning is often exacerbated by performing other management tasks at the same time. Some of these include dehorning, moving the calf into group housing, change in diet (offering different starter and/or hay), removing extra teats, etc. All of these stresses should be minimized at weaning and should be performed at other times to minimize that stress.
Dry Feeds and Rumen Development
Biological Impact of Weaning
At weaning, the calf is forced to undergo several dramatic changes. Consider the following:
At birth, the rumen and reticulum are under-developed, sterile, and nonfunctional. Liquid feeds are shunted past the reticulorumen by the esophageal groove. However, by the time the calf is weaned, the rumen is the primary compartment of the stomach. It has increased in size, metabolic activity, and blood flow to the rumen has increased. Prior to weaning, the primary source of nutrients is liquid. During the transition period, both liquid and solid feeds provide nutrients to the calf. After weaning, only solid feeds (starter and hay) are available. Before solid feed is consumed, the abomasum is the primary compartment of the stomach and both energy (glucose and fat) and protein are derived from dietary sources. However, by weaning, the rumen has become an important compartment of the stomach, and all feed consumed is exposed to bacterial fermentation prior to reaching the abomasum. A net result of this fermentation is a change in the type of energy and protein available to the calf.
Not only does the activity of the stomach compartments change, but the size of each compartment changes as well. The percent of the stomach as reticulo-rumen increases from a low of about 38% to a high of 67% by 16 weeks of age (Table 3). Note, however, that by 4 weeks of age, the reticulo-rumen has increased to 52% of the total stomach capacity. In contrast, the proportion of the stomach as abomasum declines from a high of 49% at birth to a low of 11% after 32 weeks of age. The absolute size of the abomasum does not decline; the reticulorumen simply grows at a much faster rate than the abomasum during ruminal development.
Table 3. Composition of the ruminant stomach at various ages.
| Compartment, % of total |
Age in weeks | ||||||
| 0 | 4 | 8 | 12 | 16 | 20-26 | 34-38 | |
| Reticulorumen | 35 | 52 | 60 | 64 | 67 | 64 | 64 |
| Omasum | 13 | 12 | 13 | 14 | 18 | 22 | 25 |
| Abomasum | 49 | 36 | 27 | 22 | 15 | 14 | 11 |
Adapted from Church (6).
Factors Required for Rumen Development
There are five requirements for ruminal development:
1. Establishment of bacteria in the rumen.
2. Liquid in the rumen.
3. Outflow of material from the rumen (muscular action).
4. Absorptive ability of the tissue.
5. Substrate.
A number of other metabolic changes occur during ruminal development in both the rumen and other tissues, but we will consider the above requirements for the rumen to begin to function.
Bacteria
When the calf is first born, the rumen is sterile; there are no bacteria present. However, by one day of age, a large concentration of bacteria can be found, which are mostly aerobic (or oxygen-using) bacteria. Thereafter, the numbers and types of bacteria change as dry feed intake occurs and the substrate available for fermentation changes. The change in bacterial numbers and types is almost always a function of intake of substrate (19). Prior to consumption of dry feeds, bacteria in the rumen exist by fermenting ingested hair, bedding, and milk that flows from the abomasum into the rumen. The substrate ingested will also affect the types of ruminal bacteria that flourish in the young rumen. For example, calves fed mostly hay develop a different flora from those fed mostly grain.
Liquid in the Rumen
To ferment substrate (grain and hay), rumen bacteria must live in a water environment. Without sufficient water, bacteria cannot grow and ruminal development is slowed. Most of the water that enters the rumen comes from free water intake. If water is offered to calves from an early age, this is not usually a problem; unfortunately, many producers in the U.S. do not provide free water to their calves until calves reach four or more weeks of age. Offering water in the winter can be a significant challenge in Canada and the northern U.S. However, calves still need water, even when it is cold. Sometimes, it may be necessary to bring warm water at an additional feeding to ensure that calves have enough liquid water available. Free water has been shown to increase rate of body weight gain and reduce scours (16).
Milk or milk replacer does not constitute "free water". Milk or milk replacer will by-pass the rumen by closure of the esophageal (reticular) groove. Closure of the groove is a neural response to feeding. Free water does not stimulate closure of the groove, so water enters the rumen. Feeding water can increase body weight gain, starter intake, and reduce scours score.
Outflow of Material from the Rumen
Proper ruminal development requires that material entering the rumen must be able to leave it. Measures of ruminal activity include rumen contractions, rumen pressure, and regurgitation (cud chewing). At birth, the rumen has little muscular activity and few rumen contractions can be measured. Similarly, no regurgitation occurs in the first week or so of life. With increasing intake of dry feed, rumen contractions begin. When calves are fed milk, hay, and grain from shortly after birth, rumen contractions can be measured as early as three weeks of age. However, when calves are fed only milk, rumen contractions may not be measurable for extended periods. Cud chewing has been observed as early as seven days of age and may not be related to ruminal development per se. However, calves will ruminate for increasing periods when dry feed (particularly hay) is fed.
Absorptive Ability of the Rumen Tissue
The absorption of end-products of fermentation is an important criterion of ruminal development. The end-products of fermentation, particularly the volatile fatty acids (VFA; acetate, propionate, and butyrate) are absorbed into the rumen epithelium, where propionate and butyrate are metabolized in mature ruminants. Then, the VFA or end-products of metabolism (lactate and ß-hydroxybutyrate) are transported to the blood for use as energy substrates. However, there is little or no absorption or metabolism of VFA in neonatal calves. Therefore, the rumen must develop this ability prior to weaning.
The rumen wall consists of epithelial and muscular layers. Each layer has its own function and develops as a result of different stimuli. The muscle layer provides support for the interior (epithelial layer) and moves ruminal contents in the rumen. The epithelial layer is the absorptive layer of tissue inside the rumen which is in contact with rumen contents. This tissue contains many small finger-like projections called papillae. These papillae provide the absorptive surface for the rumen. At birth, the papillae are small and non-functional. They absorb little and do not metabolize significant VFA.
Many researchers have evaluated the effect of various compounds on the development of the epithelial tissue in relation to size and number of papillae and their ability to absorb and metabolize VFA. Results of these studies indicate that the primary stimulus to development of the epithelium are the VFA, particularly propionate and butyrate. Milk, hay, and grain added to the rumen are all fermented by the resident bacteria to these acids; therefore, they contribute VFA for epithelial development. Plastic sponges and inert particles, both added to the rumen to provide "scratch", did not promote development of the epithelium. These objects could not be fermented to VFA, and thus did not contribute any VFA to the rumen environment. Therefore, rumen development (defined as the development of the epithelium) is primarily controlled by chemical, not physical means. This is further supported for the hypothesis that ruminal development is primarily driven by the availability of dry feed, but particularly starter, in the rumen.
Availability of Substrate
Bacteria, liquid, rumen motility, and absorptive ability are established prior to rumen development, or develop rapidly when the calf begins to consume dry feed. Thus, the primary factor determining ruminal development is dry feed intake. To promote early rumen development and allow early weaning, the key factor is early consumption of a diet to promote growth of the ruminal epithelium and ruminal motility. Because grains provide fermentable carbohydrates that are fermented to propionate and butyrate, they are a good choice to ensure early rumen development. On the other hand, the structural carbohydrate of forages tend to be fermented to a greater extent to acetate, which is less stimulatory to ruminal development.
Changes in Nutrients with Dry Feed Intake
As the rumen develops, there is a change in the types and amounts of nutrients available to the calf. For example, the amount of glucose that was available from intestinal digestion of lactose from milk or milk replacer is replaced by VFA from ruminal fermentation. Consequently, the amount of glucose in the blood declines and the amount of VFA and ß-hydroxybutyrate increases (Figure 5). Since glucose is the major energy metabolite in all animals, the decreased availability of glucose requires a considerable shift in "metabolic machinery" by the calf.
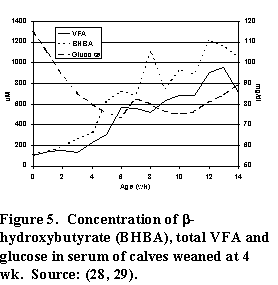
Similarly, there is an increase in the concentration of acetate and ß-hydroxybutyrate in the blood as a consequence of ruminal development. These changes are always closely associated with starter intake, which is further evidence of the relationship between intake and ruminal development.
Effect of Physical Form of the Ration
The Role of Forage
For many years, producers have fed forage, primarily hay, to calves to promote ruminal development. The common reason was to give the calf the "scratch" needed to start development of the rumen. In fact, the development of rumen function is primarily chemical and is caused by VFA in the rumen. Providing forage has less of an effect on ruminal epithelial development, thus on activity and function. The concept of "scratch" to develop the rumen is a myth. However, forage is important to promote the growth of the muscular layer of the rumen and to maintain the health of the epithelium. Rumen papillae can grow too much in response to high levels of VFA; when this happens, they may clump together, reducing the surface area available for absorption. Also, some "scratch" is needed to keep the papillae free of layers of keratin, which can also inhibit VFA absorption. Therefore, hay should be part of the diet, after weaning. A good recommendation is to wean at four to five weeks of age and offer hay from six to seven weeks of age. If calves are not weaned until eight to ten weeks of age, it may be a good idea to feed a limited amount of hay (500 grams/day) from about six weeks of age. However, the amount of hay should be limited to ensure that calves will consume sufficient starter.
There are other reasons to limit the hay offered to preweaned calves. The first is voluntary intake. Most calves do not eat significant amounts of hay if grain is also offered. Therefore, producers feed calves the best quality hay available on the farm, only to have it turned into bedding. Most of the intake of hay occurs only after six to seven weeks of age. This is a good time to put hay in front of calves.
Another reason not to feed hay to calves prior to weaning is the energy requirement of young calves. Calves have a high energy requirement relative to their ability to consume dry feed. Therefore, if calves consume significant amounts of hay, their intake of other feeds (i.e., starter) will be limited. This has the effect of reducing intake of starter, which can slow growth. Finally, most hay has too little energy for calves. The energy requirement for calves can usually be met only when calves are fed milk or high quality milk replacer, and/or excess colostrum and calf starter. Even good quality legume hay generally has too little energy to support growth of preweaned calves.
Strategies that Affect Rumen Development
There are many ways to feed calves. The protocol described here is designed to optimize rumen development, allow early weaning, and reduce the costs of the calf rearing enterprise. It should be used in conjunction with good management and close daily evaluation of calves.
As described above, the key to rumen development is dry feed intake, especially calf starter intake. To maximize starter intake for preweaned calves you should:
Weaning
Optimally, when large breed calves are consuming 700 g of starter for two consecutive days, calves can be successfully weaned. When calves are offered starter ad libitum from three days of age, this usually occurs between four and five weeks of age. However, in some situations (e.g., very cold weather or during an outbreak of disease), intake of starter by calves may not reach 700 g/day until after five weeks of age. Therefore, it is important that the recommendations of weaning at four to five weeks of age be tempered with common sense and good management to be sure that calves are not weaned before they are ready.
Most producers in the U.S. wean their calves at eight weeks of age (25). This could easily be reduced to six weeks of age with improvements in management, and to four to five weeks of age with careful management. Calves require less labor after weaning, and feed costs and costs of veterinary treatments are lower. Therefore, it is always less costly to wean calves early.
What Type of Starter?
There are many types of starters and other feeds available for calves:
There are many commercial calf starters on the market. In general, these high quality feeds are very palatable and provide nutrients required for rumen development and acceptable calf growth. In addition, many starters contain ingredients that are not normally available in lactation feeds or in home-made grind and mix starters. Unfortunately, there are also commercial starters that contain low-quality by-products and fillers that cannot support adequate growth.
Many commercial calf starters include some B-vitamin supplementation to provide calves with a source of B-vitamins before the rumen begins to produce large amounts of B-vitamins on its own. Also, many commercial calf starters contain a coccidiostat (Bovatec®, Deccox® and Rumensin® are examples) that provide protection against coccidial infection. Protection provided by a coccidiostat is inexpensive and very important, so a coccidiostat should be part of all starters in locations where coccidiosis is a management problem.
Palatability is generally highest with textured feeds, followed by complete pellets. Calves generally do not like mash feeds and palatability and intake are usually lower than with other types of feeds. Fines in pelleted calf starters can also depress intake. Look for a starter with pellets that will not break into fines. Lactation feeds generally should not be fed to calves. In many cases, lactation complete feeds do not contain sufficient energy to support good calf growth. Also, lactation feeds may contain added fat, animal by-products, or other additives that reduce palatability. Finally, lactation complete feeds do not contain added B-vitamins or coccidiostat.
Housing
There are numerous methods of housing calves. Regardless of the system or method of housing used, there are certain requirements for all calf housing. These include ventilation, isolation, comfort, and economy. Major criteria are to keep calves dry, free from drafts, away from pathogens, and to minimize costs associated with calf housing.
Ventilation is critical to successful calf rearing. Calves are especially susceptible to infection caused by bacteria and viruses, and aerosols produced by sneezing and coughing should be removed from the area as soon as possible. Proper ventilation also removes noxious gases and humidity which can stress animals, thereby reducing their resistance to disease and causing respiratory problems.
Isolation minimizes the contact among calves, thereby reducing the spread of disease-causing pathogens. Typically, isolation is accomplished by using individual calf housing such as hutches, which physically separate animals. Isolation also helps to promote observation of the health of individuals. Calves should be isolated until about two weeks after weaning, when they can be moved into small groups of similarly sized calves.
Comfort implies access to feed and water, a dry environment, and control of extremes in temperature. Access to feed and water should be convenient for consumption by the animal and delivery by the producer. It is especially important to minimize the moisture in the environment. Calves are able to tolerate cold temperatures much better if they are dry and well bedded. However, when calves become wet, the insulative value of hair and bedding are markedly reduced, making them much more susceptible to chilling. Drainage, selection of bedding materials, and frequency of cleaning are important criteria for minimizing the moisture of calf housing.
Economy in calf housing is essential to minimize expenditures without tangible benefit. In most cases, loss of money in calf housing is due to excess costs of warm housing in areas that don't require it, and the false economy of providing less than optimal housing for calves. Because calves are especially sensitive to environmental stresses, it is especially important to provide them a clean, dry, comfortable environment. The alternative is morbidity and mortality.
Summary
Raising calves from birth to weaning must be directed toward the acquisition of passive immunity through proper colostrum feeding, feeding liquids and calf starter to ensure a smooth transition from preruminant to ruminant digestion, and housing to minimize stress and spread of pathogens. With proper management, it is possible to minimize death loss of young calves (< 5% of calves born alive) and thereby improve profitability of the replacement enterprise.
References