Influence of Monensin on Post-Partum Health and Production
Department of Population Medicine, Unviversity of Guelph, Guelph, ON, Canada N1G 2W1
#
Take Home Messages# Introduction
The first 10 to 12 weeks of lactation (early lactation) place extreme demands on the modern dairy cow. During this period a cow must adjust from consuming a high fiber dry cow ration, to a lactating cow diet high in energy and relatively low in fiber. The drive for increased milk production draws on body fat stores to compensate for inadequate energy intake. The resulting increased mobilization of free fatty acids leads to the synthesis and accumulation of ketone bodies.
Elevated levels of ketone bodies (beta-hydroxybutyrate (BHB), acetone, and acetoacetate) lead to a condition called ketosis. Clinical signs of ketosis include: weight loss, decreased appetite (especially for concentrates), firm dry manure, and a noticeable decrease in milk production. Increased circulating levels of ketone bodies without the presence of clinical signs is referred to as subclinical ketosis. Both clinical and subclinical ketosis are considered varying degrees of the same condition.
Since ketosis occurs early in lactation, prevention has focused on nutritional management of the dry and transition cow. Avoidance of ketogenic feedstuffs and increased frequency of feeding concentrates have been advocated as preventive measures for subclinical ketosis. Avoiding overconditioned cows in late lactation and the early dry period, as well as lead feeding with concentrates about three weeks prior to calving have also been suggested as aids in prophylaxis. In addition to good nutrition, certain feed additives have been helpful in preventing subclinical ketosis. Daily supplementation with niacin fed prior to calving was shown to reduce circulating levels of BHB in one herd of lactating dairy cows. Propylene glycol has been found beneficial when administered prophylactically, however, its use requires daily oral administration. Sodium propionate was helpful in preventing clinical ketosis but reduced feed intake. Monensin has also been proposed as a potentially useful antiketogenic agent.
Monensin administered in the feed was effective in alleviating a herd problem of clinical ketosis (Rogers & Hope-Cawdery, 1980), and in reducing levels of circulating total ketone bodies (Sauer et al, 1989). There have been relatively few reports on the influence of monensin or lasalocid on milk production and milk components in North American dairies. Only recently has there been published research describing the influence of monensin on reproductive performance in lactating dairy cows. There is also a lack of clinical trial data addressing the role of monensin on periparturient disease.
The primary objective of this field study was to investigate the efficacy of a monensin CRC (Rumensin
7 CRC, Provel, a division of Eli Lily Canada, Research Park Centre, 150 Research Lane, Suite 120, Guelph, Ontario, Canada), administered prepartum, for the prevention of subclinical ketosis in lactating dairy cattle. A second objective for this study was to evaluate the impact of monensin on milk production and milk components, cow health, and reproductive performance.# Materials and Methods
Study Design
Twenty-five Holstein dairy herds located within 30 kilometers of Guelph, Ontario, Canada were selected, based on the herd operators
= willingness to participate in the study, and the herds= enrollment in Ontario Dairy Herd Improvement Corporation (DHI) for the recording of milk and component measurements. Assignment of monensin CRC or placebo capsules was randomized within farm using random number tables and the CRC was administered approximately three weeks prior to the expected calving date. The monensin CRC is a sustained release intraruminal device administered per os that contains 32 grams of monensin in a hexaglycerol distearate matrix core. In mature Holstein cows, it delivers approximately 335 mg of monensin sodium per day for an average of 95 days. Plastic wings at one end of the capsule are present to help prevent regurgitation. The placebo capsule was identical in all respects except it contained no monensin. First lactation animals were excluded if any ionophore was fed to heifers within 4 weeks of parturition. Five farms participated with first lactation animals excluded on this basis. Herd size ranged from 25 to 160 milking Holstein cows and the rolling herd average for milk production was between 7000 to 10000 kg. Five herds fed total mixed rations while the remaining 20 farms had component feeding systems. Four herds had freestall barns and the others used tiestall facilities. A field study technician administered the assigned treatments, collected, prepared and submitted the blood and milk samples, and assisted with the database management. All farm personnel, veterinarians, and researchers were blinded to the treatment. All capsules had a unique four digit code which was recorded for every animal treated. Both monensin and placebo capsules were re-administered in cases of regurgitation prior to capsule payout. If the capsule was damaged, a new bolus corresponding to the same treatment was given.Sampling
Every farm was visited weekly in the morning, on the same day of the week and at approximately the same time of day. Blood was collected at the time of capsule administration and during weeks 1, 2, 3, 6, and 9 postcalving. Cows were body condition scored on a scale of 1 to 5 using 0.25 increments at the time of each sample collection. Composite milk samples were obtained from each cow at the same time as blood collection postcalving. Blood samples were submitted to the Clinical Pathology Lab, Department of Pathobiology, Ontario Veterinary College for the determination of calcium, phosphorus, total protein, urea, glucose, BHB concentration, and aspartate aminotransferase (AST) activity. Milk ketones were measured using a commercially available sodium nitroprusside based test (KetoCheck). Any colour change (pink or purple) was recorded as a positive reaction. Milk containing blood was not evaluated because this hindered the colour change interpretation.
Data Management and Statistical Analysis
Laboratory data, cow reproductive and health information, and DHI test results were stored in microcomputer databases linked by unique cow identifications. The data were checked for errors compared with written reports and outliers were rechecked to be sure values were accurate. Analysis of data was conducted primarily using repeated measures analysis of variance for continuous data (laboratory and milk production) and logistic regression for binary data (health).
# Results
Laboratory Data
A total of 503 cows were given monensin CRC
=s and 507 received placebo capsules. Approximately 28% were in first lactation, 28% in second lactation, and the remainder were third parity or greater. Over 60% of the cows calved in the spring and summer, and 68% were classified in fair body condition prior to calving.The levels of BHB, glucose, AST, urea and body condition score were significantly influenced by treatment. There were no significant treatment effects found for calcium, phosphorus, and total protein.
Beta-hydroxybutyrate was significantly lower for monensin-treated cows in weeks 1,2, and 3 postcalving. The magnitude of this difference was a decrease in mean BHB values of 200 to 300
Fmol/L in monensin-treated cows. Glucose was significantly increased in monensin-treated cows during weeks 1 and 2 postcalving. This difference reflected an increase in serum glucose of 0.15 to 0.20 mmo1/L. Serum urea was increased significantly in the monensin CRC group during weeks 2 and 3 postcalving. Mean concentrations of serum urea were 0.4 to 0.5 mmol/L higher than placebo cows for weeks 2 and 3 postpartum, respectively.Monensin treatment reduced AST activity during the entire postcalving period. Treatment with monensin also had a significant effect on reducing body condition loss postcalving. The mean body condition scores were significantly higher for monensin-treated cows during the entire postpartum period. However, by week nine the difference amounted to less than 0.1 of a body score.
Monensin treatment reduced both the prevalence and incidence of subclinical ketosis by about 50%. This effect appeared to be primarily for the first two weeks postcalving (Figure 1). In total, there were 456 cows that had subclinical ketosis in early lactation. Treatment significantly reduced the mean number of serum samples at, or above, the 1200
Fmol/L BHB threshold by 0.4 tests per cow. Monensin treatment also had a significant protective effect on the percent of cows showing positive milk ketones.Figure 1.Incidence of Subclinical Ketosis Defined by a Serum Threshold of
$ 1200Fmol/l Beta-hydroxybutyrate in Dairy Cows that Received Either a Monensin or Placebo Controlled Release Capsule at Three Weeks Prepartum.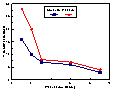
Note: Incidence of subclinical ketosis significantly different at P <0.01.
Milk Production and Milk Components
Data on a total of 952 cows were available for the analysis of the treatment effect on test day milk production and milk components. The effect of treatment was not significant for cows classified as being thin (BCS < 3.25). Treatment with monensin had a statistically significant positive effect on milk production at the second DHI test in fair conditioned cows (P =0.014). Monensin treatment significantly increased milk production for all three DHI tests (P =0.03) in cows classified as fat (BCS
$4.0). Graphs of actual milk production by treatment and DHI test number for each body condition class are illustrated in Figures 2, 3, and 4.No significant treatment effect was found for either milk fat percent or milk protein percent.
Figure 2. DHI Test Day Milk Production (kg) by Treatment (Monensin or Placebo) for Cows Categorized as Thin (Body Condition Score <3.25) at the Time of Treatment Administration (approximately 3 weeks prior to parturition).
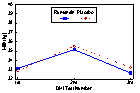
* No statistically significant difference in milk yield between treatments.
Figure 3. DHI Test Day Milk Production (kg) by Treatment (Monensin or Placebo) for Cows Categorized as Fair (Body Condition Score 3.25 to 3.75) at the Time of Treatment Administration (approximately 3 weeks prior to parturition).
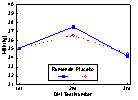
* Milk yield significantly different at 2nd DHI test (P <0.05).
Figure 4. DHI Test Day Milk Production (kg) by Treatment (Monensin or Placebo) for Cows Categorized as Fat (Body Condition Score >3.75) at the Time of Treatment Administration (approximately 3 weeks prior to parturition).
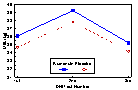
* Milk yield significantly different for the entire period (P <0.05).
Crude lactational incidences and median days to diagnosis for all health events are displayed in Table 1.
Monensin-treated animals were 40% less likely to experience an abomasal displacement than placebo animals (P = 0.004). Animals in first and second lactation were less likely to incur abomasal displacement than cows in greater parities. The occurrence of a retained placenta increased the risk of displacement by a factor of two. Cows categorized as fat prior to calving were at three times greater risk of developing clinical ketosis than cows in fair condition at the time of capsule administration. Thin cows were not at significantly greater or lesser risk of ketosis than fair conditioned cows. Treatment with monensin was not statistically significant in reducing the incidence of clinical ketosis (P =0.11), however the point estimate of the odds ratio was 0.47. Monensin treatment prior to calving significantly reduced a cow
=s risk of developing more than one disease in the peripartum period. The occurrence of twins, season of calving, parity and body condition at treatment administration also significantly influenced the risk of multiple disease events. Cows calving in the summer months were at increased risk of multiple illness. First and 2nd lactation animals were less likely to have multiple illness than those cows in 3rd and greater lactations. Cow that were in heavy body condition were at two times greater risk of incurring multiple disease events. There was a tendency for monensin treatment to reduce the risk of a cow being sold (P =0.09).Table 1. Incidence of Disease in Holstein Cows treated with a Monensin or Placebo Controlled Release Capsule Three Weeks prior to Expected Calving Date.
|
Health Variable |
Monensin |
Placebo |
All Cows |
|
|
Incidence |
Incidence |
Median Days from Calving to Diagnosis |
|
Sold* |
8.0 |
11.6 |
24.0 |
|
Died |
2.2 |
3.2 |
7.0 |
|
Regurgitated Capsule |
4.5 |
6 |
-1.5 |
|
Twins |
5.0 |
3.4 |
0 |
|
Calving Problem |
2.6 |
3.3 |
0 |
|
Retained Placenta |
7.4 |
8.9 |
1 |
|
Milk Fever |
7.8 |
8.1 |
0 |
|
Metritis |
4.0 |
4.1 |
7 |
|
Ketosis* |
1.0 |
2 |
12 |
|
Abomasal Displacement** |
3.0 |
5.3 |
11.5 |
|
Digestive |
1.4 |
1.8 |
5.5 |
|
Mastitis |
15.1 |
17.4 |
9.5 |
|
Pneumonia |
1.0 |
1.4 |
12 |
|
Endometritis |
2.8 |
2.8 |
23 |
|
Teat Injury |
1.4 |
1 |
13 |
|
Lame |
2.2 |
2.8 |
37 |
|
Other Disease |
1.4 |
2.2 |
12 |
|
Disease |
35.4 |
37.4 |
N/A |
|
Multiple Illness** |
8.9 |
13.2 |
N/A |
**Treatment incidence significantly different at P
# 0.05* Treatment incidence significantly different at P
# 0.12Reproductive Performance
Conception rate was not significantly influenced by treatment. Treatment had no significant effect on the interval from calving to first breeding. Similarly treatment had no significant effect on the interval from calving to pregnancy. There was no statistically significant difference (P =0.65) in the lactational incidence of cystic ovaries between placebo cows (2.0%) and monensin-treated cows (1.6%).
# Discussion
Serum Biochemistry and Body Condition Scores
Administration of a monensin CRC to dairy cows three weeks prepartum significantly reduced serum BHB concentrations and other measures of energy balance, as compared to placebo-treated control cows. The observed higher levels of glucose during weeks 1 and 2 postpartum is the first report of monensin significantly improving serum glucose concentrations in lactating dairy cattle. Monensin could influence BHB and glucose levels in several ways. Monensin is known to increase propionic acid and reduce butyric acid in the rumen. Higher concentrations of propionate would result in more glucose production via the citric acid cycle. Increases in glucose might stimulate elevations of insulin levels which could reduce fatty acid mobilization, thereby decreasing ketogenesis. A reduction in rumen methane production by monensin might increase the efficiency of digestion. This may indirectly provide more glucose to the animal compared to untreated animals consuming the same feed.
Higher urea levels have been previously reported for monensin-treated dairy cows (Hayes et al, 1996). This is consistent with the current results which found significantly increased levels of urea in weeks 2 and 3 postcalving. The mean values of urea for both treated and placebo animals in this study are within suggested reference ranges for lactating cows (Nelson, 1995).
Aspartate aminotransferase has not been measured in other lactating cow monensin trials. The decrease of AST activity in monensin treated cows postcalving support the beneficial findings of increased glucose and reduced BHB concentrations. Although sources for increased AST concentrations are not specific to liver damage, the observed lower serum activity of the enzyme in monensin-treated cows may demonstrate some improvement in liver function in these animals.
Mean body condition scores were significantly higher in monensin-treated cows postcalving. By week nine postcalving, monensin-treated cows were 0.1 BCS points higher than placebo animals. This difference is obviously not clinically detectable, and may reflect statistical rather than biological significance. However, it certainly suggests that there was no additional loss of body weight in monensin-treated cows compared to placebo animals. In addition, it lends support to the hypothesis that monensin reduced fatty acid mobilization through a reduction in BHB levels and increased glucose concentrations.
Monensin
=s effect on the incidence or prevalence of subclinical ketosis has not previously been reported. Using the threshold of 1200 Fmol/L BHB, defined a priori, the risk of subclinical ketosis was reduced by approximately half when cows received a monensin CRC three weeks prepartum. This risk was reduced by the same magnitude for both incidence and prevalence.Since monensin influenced both the incidence and prevalence of subclinical ketosis, and prevalence is a function of both incidence rate and duration, monensin likely had an effect on the duration of the condition. Exact measurement of duration was not possible because of the frequency of serum collection in this study. However, the number of positive ketone tests postcalving using serum BHB
$ 1200 Fmol/L, gave a crude estimate of duration. Treatment significantly reduced the duration of disease by 0.4 fewer positive tests per cow.Milk Production and Milk Components
Monensin treatment exerted a positive influence on milk production, however, the response depended on the cow
=s initial body condition prior to calving. Dairy Herd Improvement test day milk yield was not significantly influenced by treatment in cows categorized as being thin at the time of treatment administration. Cows in fair body condition given monensin produced significantly more milk at the second DHI test compared to placebo cows. This amounted to an actual difference of 0.85 kg more milk per day at the second DHI test and an adjusted difference of 1.2 kg per day using least squares means. The second DHI test occurred at an average of 60 days postpartum and would be the closest measurement to peak milk yield. It has been estimated that for every 1 kg increase in peak milk yield, about 200 kg more milk is produced during a lactation. Although there were no statistically significant differences in projected 305 milk yield, monesin treated cows had 106 kg more projected lactation milk yield than placebo cows (using least square means). Milk yield in fair conditioned cows at both first and third DHI test were not significantly different between treatments. Since the average time from treatment to calving was 21 days, and the capsule is empty at approximately 95 days, the daily release of monensin would have ceased at about 74 days postpartum. This is half way between the 2nd and 3rd DHI test. It is possible that a second monensin capsule administered at day 75 postcalving may have continued to improve milk production. Cows treated with monensin and classified as fat prior to capsule administration produced significantly more milk than fat cows receiving the placebo. The difference amounted to an additional 1.2 kg per day unadjusted and 2.5 kg per day using least squares means to control for other factors in the model. The response in unadjusted milk yield observed in this study was a 3.4% increase for fair cows at peak lactation and 7.4% for fat cows during all three DHI tests. This compares to the 7 to 8% increase found by Lynch et al (1990) and the 6% increase reported by Lowe et al (1991). In absolute kilograms of milk, the increase of +0.85 kg per day found for fair cows at peak and 1.25 kg per day for fat cows during the first 94 days of lactation is very similar to the 1.1 kg per day increase observed by Lowe et al (1991) and the 1.0 kg per day increase reported by Lynch et al (1990).Other studies have not identified the monensin by body condition score interaction observed in this trial. It is unclear why there appears to be a dose response type effect with monensin and body condition prior to calving. Heavier body condition prior to calving appeared to favour a greater production response to monensin. From the BHB data, it was shown that levels of BHB increased postcalving with increasing initial body condition score class. Although monensin consistently reduced BHB levels for all body score classes, perhaps heavier body score classes have concentrations of BHB approaching a subclinical ketosis threshold. The observed increase in milk production may simply be the result of an improved energy supply that alleviates the negative impact of subclinical ketosis on milk production.
The treatment effect on test day milk fat percent and test day milk protein percent in the current study differ from the findings of other studies which indicated that monensin significantly lowered milk fat percent (Lynch et al, 1990; Lowe et al, 1991; Abe et al, 1994; Hayes et al, 1996). These studies involved pasture-fed cattle, whereas the cows in the current project were fed a mix of concentrate and dry or ensiled forage (which is more representative of North American lactating cow rations). Phipps et al. (1995) found a dose relationship between monensin levels and milk fat, with higher monensin levels causing greater fat depression. It may be that the daily dose of monensin provided by the capsule was not sufficient to influence either milk fat percent or milk protein percent.
Health
A monensin CRC given prepartum appeared to improve cow health in several ways. First, monensin was associated with significantly less risk of displaced abomasum. The odds ratio estimate of treatment on the risk of abomasal displacement was 0.59 suggesting that monensin decreased the risk of displacement by 41%. This may also partially explain the trend of less cows sold in the monensin group since abomasal displacement increased the risk of being culled. The observed treatment effect in this trial supports previous reports which have suggested that ketosis increases the risk for abomasal displacement ( Curtis et al, 1986; Grohn et al, 1989). Monensin likely exerts its protective effect on reducing the incidence of abomasal displacement through antiketogenic properties, however, there may be other properties of monensin such as reduced methane production and reduced lactic acidosis that could potentially influence the risk of displaced abomasum. Retained placenta increased the risk of abomasal displacement by two times. Therefore, strategies to reduce the incidence of abomasal displacement also should include reducing the incidence of retained placenta. The risk of displacement was significantly lower for first and second lactation animals compared with third or greater lactation animals.
Treatment prepartum with a monensin CRC tended to reduce the risk of clinical ketosis (P =0.11). The lack of a treatment effect at P
#0.05 is likely a sample size issue since the disease incidence was relatively low with 10 cases of ketosis in the placebo group and only 5 cases in the monensin group. A larger sample size would be required to definitively prove there is a protective effect of treatment on reducing the incidence of ketosis. The serum biochemistry findings of reduced BHB, increased glucose, reduced AST levels, and both a reduced incidence and prevalence of subclinical ketosis strongly support that monensin reduced the risk of clinical ketosis. It is interesting to note that body condition prior to calving was a significant factor in the model. The risk of clinical ketosis was three times greater for fat cows than for thin or fair conditioned cows. The variable multiple illness which represented animals diagnosed with more than one disease was created to test the effect of monensin on disease complexes, since most periparturient diseases are interrelated (Curtis et al, 1986; Grohn et al, 1989). Monensin reduced the risk of multiple illness by 40% (P =0.01). The current study design did not allow determination of which disease complexes monensin was helpful in preventing because of the relatively low incidence rates of most periparturient disease. Given that monensin appeared helpful in preventing both ketosis and displaced abomasum, one would expect these diseases to play a primary role in the prophlyactic effect of monensin on reducing the incidence of disease complexes. However, even with both ketosis and displaced abomasum removed (having accounted for 20% and 36% of multiple illness for monensin and placebo cows respectively), there was still a lower incidence of multiple illness in monensin-treated cows (7.4% in monensin vs 9.1% in placebo animals). There was no statistically significant difference in the absolute number of cows with a periparturient disease between monensin and placebo treatments. This suggests that cows treated with monensin precalving still developed illness, but were less likely to develop multiple diseases in early lactation compared to controls. Further work with a larger data set will be necessary to clarify these protective effects of monensin on periparturient disease.There was some indication that monensin reduced the risk of being sold in the first 94 days of lactation. Treatment was significant at P<0.10 but not P<0.05, suggesting that there may be an effect of monensin but further work is necessary to investigate this potentially beneficial effect. One explanantion for this trend might be that higher milk production in monensin-treated cows, reduced the risk of being sold. In addition to treatment, cows with abomasal displacement were 3.8 times more likely to be sold and cows with a teat injury were at nearly six times greater risk of leaving the herd.
Reproductive Performance
The findings that monensin treatment had no significant effect on reproductive parameters is consistent with previous research (Hayes et al, 1996; Lean et al, 1994); although none of the trials exactly duplicate the methodology used in the current study. Although monensin treatment reduced BHB levels, monensin may not impact on energy balance sufficiently to be helpful in improving reproductive performance.
#
ConclusionsAdministration of a monensin controlled release capsule prepartum had a marked positive influence on energy indicators in early lactation. Monensin decreased BHB concentrations and increased both glucose and urea levels. Monensin also reduced body condition score loss and reduced serum AST activity. The prevalence, incidence and duration of subclinical ketosis were all significantly reduced by monensin treatment.
Prepartum monensin treatment also improved both health and milk production in lactating Holstein cows and first parity heifers. The influence of monensin on milk production was dependent on initial body condition score at the time of treatment administration; with a higher production response observed with greater body condition. There was no effect of monensin on milk fat or milk protein percent. Monensin significantly reduced the risk of both abomasal displacement and multiple illness. Monensin tended to reduce the risk of clinical ketosis and being sold. There were no significant effects of monensin noted for any reproductive outcome.
#
References