Buffers - What and When to Use
Department of Animal and Dairy Science, University of Georgia, Tifton Campus, Tifton, GA 31793-0748 U.S.A
Take Home Messages
Introduction
Dietary buffers are compounds which were originally added to the diets of dairy cows to improve milk fat content. More recently buffers are recognized to minimize wide fluctuations in rumen pH postfeeding which leads to improved digestion of fiber, increased acetate:propionate ratio in the rumen (which improves milk fat percentage), and often greater feed DMI as well as milk yield. Dietary buffers have been very well researched and are widely used in the dairy industry. The need for buffers developed because of changes in the way dairy cows are fed. Buffers are used largely to offset the acidic conditions produced by the relatively high grain rations fed today. In the past when cows were fed large quantities of forage and relatively small amounts of grains buffers were not needed. However, genetically superior dairy cows capable of high milk yield required increasing nutrient density (especially energy) in the diet which was supplied to a great extent by larger quantities of grains. Increased reliance on finely chopped, fermented feeds further increased the challenge to maintain rumen pH in the absence of sufficient rumination and greater acidity of the feedstuffs.
In nutrition the term buffer is applied loosely to several compounds, including bicarbonates, carbonates, hydroxides, and oxides. The efficacy of some compounds described as buffers is well established, while for others less is known, or the effectiveness has not been proven. The field conditions under which buffers are most effective have been well established. However, these conditions are sometimes unknown to the producer, resulting in inappropriate or ineffective use.
The objectives of this paper are to establish the basis for the need of buffers, discuss types of buffers, and establish some situations where buffers may, or may not, be most effective.
Ruminal Responses to Modern Dairy Diets
Maintenance of an acceptable rumen pH is critical to good performance as well as health of the dairy cow. The range of pH which is desirable for good rumen function is about 6.4 to 6.8. Deviations from this range, especially on the acidic side, have negative implications for the rumen microbe population. Low rumen pH alters the rumen volatile fatty acid pattern, reduces milk fat percentage, can reduce intake, and alters microbial populations by reducing the number of fiber digesting bacteria. The fermentation of feedstuffs by rumen microbes results in acid production, and feedstuffs such as silages also contribute acidity which must be neutralized or buffered to maintain pH. The cow has three primary means of buffering these acid sources: 1) buffers contained in the saliva, 2) inherent buffering capacity of consumed feeds, and 3) buffers added to the cow's diet.
The cow secretes saliva during chewing and rumination, as well as at rest. The quantity of saliva secreted by lactating dairy cows may range from about 130 to over 300 liters per day, and total secretion of sodium bicarbonate in saliva is estimated to be about 3500 grams per day (5). The rate at which saliva is secreted depends only slightly on the feedstuff being consumed, but depends greatly upon how long it takes to consume (and ruminate) the particular feed. The eating rate of forages (grams feed/minute) is much slower for hays than for a pelleted ration, while silage and fresh grass are intermediate (23). Consequently, the quantity of saliva secreted per gram of feed consumed is much greater for long stem, fibrous feeds such as hays.
The quantity of saliva generated is critical because of the amount of buffering present. Saliva is rich in buffers (primarily bicarbonates and phosphates). Based on research data, Erdman (5) calculated that, in diets where the forage portion consisted of 50% corn silage and 50% alfalfa haylage, a reduction in the forage content of the ration from 70 to 30% of diet dry matter (DM) resulted in a 199 gram/day decrease in sodium bicarbonate (NaHCO3) flow. This reduction is equivalent to adding .75 to 1.0% sodium bicarbonate to the diet. In addition, in diets containing 14% ADF, 20 gr of magnesium oxide or 44 gr of sodium bicarbonate was equivalent to increasing the dietary ADF content by 1 percentage unit. It is clear that the manner in which we feed has contributed to the need for buffers, and that dietary buffers can substitute, at least partially, for dietary fiber.
Many modern feeding practices favor the use of dietary buffers. These practices include: 1) feeding large quantities of diets with a high content of fermentable carbohydrate, 2) the use of forages with a high acid content (silages), 3) the tendency to feed marginal fiber diets, 4) the use of forages with small particle sizes, 5) far less use of long stem hay, 6) "slug-feeding" of concentrates twice daily, 7) the extensive use of feed by-products which have small particle sizes.
Effect of Forage and Fiber on Buffering
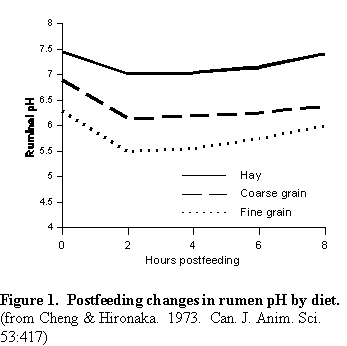
Dairy rations have changed from primarily forage to a very high content of grains. A decrease in fiber intake is associated with greater quantities of fermentable carbohydrate and a greater volatile fatty acid (VFA) production rate, especially two to three hours post feeding. The quantity of fiber consumed and the particle size which must be chewed and ruminated has also declined. Thus the total saliva yielded per kg of feed consumed has declined proportionally. The result of these feeding practices is illustrated in Figure 1, which shows the decline in rumen pH following a meal, and the effect that feedstuffs have on pH. Note that rumen pH is very high for hay diets, while for the fine grain diet pH is well below 6.0. This is very undesirable and contributes to impaired rumen digestion and low milk fat percentage. A positive linear relationship between the dietary content of ADF and rumen pH was reported (5), and digestion of ADF was improved by 3.6 percentage units per .1 unit increase in pH. Altering rumen pH between 6.0 and 6.8 has little effect on VFA production in the rumen. However when rumen pH is at 5.0 there is essentially no NDF digestion, at pH 5.5 digestion is less than 20%, and at pH 6.5 digestion exceeds 40% (20). Increasing dietary ADF in the range of 11 to 23% increased rumen acetate:propionate (A:P) ratio from about 1.5 to 3.5 (5). The increasing A:P ratio increased milk fat percentage from about 2.5% to about 3.7%. Thus adequate fiber helps to maintain ruminal pH and fiber digestion, and increases A:P ratio, increasing milk fat percentage.
Due to differences in cell wall matrix and mineral content, feedstuffs have inherent buffering characteristics which differ by species. Cation exchange capacities (CEC) of forages are compared in Table 1. Note that the CEC is greatest for alfalfa hay while that for corn silage is substantially lower. The lowest is for bermudagrass, a perennial grass grown in the sunbelt region of the U.S. Because of the wide range of CEC among these forages, the quantity necessary to be equivalent to 100 grams of calcium carbonate is slightly more than 10 kg for alfalfa hay, over twice that for corn silage. Thus alfalfa has much greater inherent buffering than corn silage or bermudagrass. This serves to illustrate that differing forages may have differing requirements for buffering upon feeding. In addition, this does not consider the effects of particle size and chewing on rumen buffering.

Table 1. Cation exchange capacity (CEC) values for forages and intake required to yield equivalent CEC capacity of 100 grams calcium carbonate.
|
Forage |
% CWC1 |
CEC2 |
CaCO3 equivalent3 |
|
|
kg CW |
kg as fed DM |
|||
|
Coastal bermudagrass |
70.0 |
11.4 |
17.5 |
25.0 |
|
Ryegrass |
41.0 |
24.4 |
8.2 |
20.0 |
|
Alfalfa hay |
53.0 |
35.6 |
5.6 |
10.6 |
|
Corn silage |
43.7 |
19.6 |
10.2 |
23.4 |
Adapted from McBurney et al. 1981. Proc. Correll Nutr Conf.
1
Cell wall content.2
Cation exchange capacity (meq/gr).3
Equivalent to the CEC of 100 gr. CaCO3, assuming 1998.4 hydrogen milliequivalents per 100 gr CaCO3.Erdman (5) suggests that total ash and cation contents of forages are good indicators of total buffering capacity, meaning that total feed ash may serve as a potential means of predicting buffering effects.
Because of these differences in forage buffering capacity, one might expect that differences exist in response to dietary buffers. Indeed, in several experiments in which sodium bicarbonate was the buffer used in diets based on alfalfa hay, there was no effect of sodium bicarbonate on DMI or milk yield (1, 4, 11), long or short particle size of alfalfa had no effect on response to buffer (16), and there was no effect of sodium bicarbonate upon digestion of dietary fiber components (3, 4, 16)
In contrast, it appears that diets with a very high proportion or corn silage may benefit from the addition of buffers. Early lactation cows switched immediately postpartum to a 40% corn silage, 60% concentrate diet peaked 2 to 3 wk earlier in intake than controls, and consumed an average of 2.1 kg per day more DM (6). The cows in this study that were offered both sodium bicarbonate and magnesium oxide averaged 3.8 kg per day more milk than controls. Levels of buffer were relatively high (1.5% sodium bicarbonate, .8% magnesium oxide), more than would be recommended today. The point of this work is that the buffers were effective, and used in early lactation apparently helped cows get on feed quicker and peak higher in both milk yield and DMI. Numerous studies with sodium bicarbonate used in diets consisting predominantly of corn silage and concentrates indicate that the buffer improves rumen pH, DMI, milk fat percentage, and milk yield.
What are Dietary Buffers
Buffers are compounds which in an aqueous solution help to resist changes in pH when acid or base are added to the solution. Many of the compounds that are used today do not meet the strict definition of a buffer, because they not only buffer but they aggressively neutralize acidity. Be that as it may, a number of compounds are used in the modern dairy industry. Sodium bicarbonate (NaHCO3) is one of the most widely used buffers on the market. Magnesium oxide (MgO) has some of the same effects as sodium bicarbonate (increased rumen pH, elevated milk fat test) but is not truly a buffer. Sodium sesquicarbonate is a mixture of sodium bicarbonate and sodium carbonate (Na2CO3), and because it contains sodium carbonate is also a neutralizer of acidity. Trona ore is an unrefined compound that contains sodium bicarbonate, sodium carbonate, and some impurities. Although the impurities may dilute the buffering agents somewhat the compound is an effective buffer. There also are multielement buffers which contain neutralizing agents and buffers. In addition to sodium bicarbonate and sodium carbonate, potassium bicarbonate and potassium carbonate have been used as buffers, although cost is often a limiting factor.
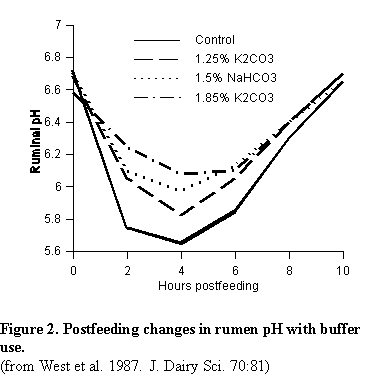
Buffers function by minimizing the change in rumen pH following a meal. Figure 2 shows how buffers moderate rumen pH changes following a meal. In this study,sodium bicarbonate and two levels of potassium carbonate were used (25). Note that sodium bicarbonate and the high level of potassium carbonate prevented rumen pH from dropping below 6.0. Ruminal pH for the control diet was below 5.7, low enough to cause depressed digestibility and low milk fat test. Addition of buffers improved milk fat percentage and fiber digestion considerably.
Erdman (6) found that the effects of sodium bicarbonate and magnesium oxide were additive, though Staples and Lough (21) reported in a summary that most studies did not show additive effects. Although both buffers are effective, several studies show that at adequate levels there was little benefit to the addition of both. However, they are often used together, and a common use level is .75 to 1.0% of diet DM for sodium bicarbonate, and .25 to .4% for magnesium oxide. This would be in the range of 160 to 210 gr of sodium bicarbonate, or 50 to 85 grams of magnesium oxide per cow daily.
Compounds which are similar to sodium bicarbonate such as sodium sesquicarbonate, sodium carbonate, and trona ore (the parent material for many of these purified compounds) have a similar chemical make up and would be expected to perform similarly based on similarities in chemistry. The number of scientific trials are far less numerous than those performed with sodium bicarbonate, but there are good comparisons in existence. Staples and Lough (21) summarized this area and reported that sodium sesquicarbonate performed as well as sodium bicarbonate, and unrefined sodium sesquicarbonate (trona) produced mixed results in several trials, although it was nearly as effective as sodium bicarbonate in several trials. The authors concluded that some additional refining of the trona might be necessary to increase its efficiency. A multielement buffer containing sodium and potassium chlorides and magnesium and sodium carbonates increased milk fat percentage and yield similarly to sodium bicarbonate when fed at 1% of the diet, and when fed at high levels (up to 3% of DM) improved fat test even further. Although beneficial the product apparently demonstrated no advantages over sodium bicarbonate.
Of course one of the keys to successful use of a product is adequate consumption. Early studies where high concentrations of buffer were fed often resulted in reduced feed intake. Sudden addition of 1.5% of sodium bicarbonate to the concentrate portion of feed reduced intake immediately, but a more gradual addition over a three week period prevented the intake decline (7). The intake decline that occurred with sudden addition of buffer was temporary. Declines in feed intake which are often associated with the use of buffers in a concentrate which is fed separately from forages can be minimized by gradual addition of the buffer to the grain. Use of a total mixed ration, and especially the use of fermented forages (silages), helps to mask flavors and sudden ration changes have much less impact on cow performance. Some compounds such as magnesium oxide can be very unpalatable and care should be taken to avoid excessive quantities in the diet, and to adapt cows to the buffer. Again, use of silages and total mixed rations helps greatly.
Feeding Studies with Dietary Buffers
The apparent contrast between forage sources and the effectiveness of buffers is an important consideration when deciding if buffers are to be used in the dairy diet. In an extensive review of the scientific literature, Erdman (5) found that on average, in 17 studies where corn silage was the sole forage fed primarily to early lactation cows, use of sodium bicarbonate resulted in a .5 kg increase in DMI and 1.1 kg increase in fat-corrected milk (FCM). Greater FCM resulted from a .6 kg increase in milk yield and .16% improvement in fat test. However, in 8 studies where a mixture of corn silage and alfalfa haylage or grass silage was fed differences were much smaller and not significant (5). There was essentially no effect where haycrop or grass silage were the sole forages (3 studies), or where alfalfa hay was the sole forage (8 studies).
A review (21) summarized studies with sodium bicarbonate since 1980. Of 28 studies where corn silage was the primary forage, cows in early lactation consuming diets supplemented with sodium bicarbonate (18 studies) produced .8 kg more milk with .16% greater fat test, equal to a 1.4 kg increase in 4% FCM yield. Cows in mid-lactation (10 studies) increased milk yield by .9 kg daily with .3% greater milk fat content, improving FCM by 1.9 kg. The authors reported that in 4 studies where alfalfa was fed as the primary forage, only one study resulted in improved FCM yield. In addition, in four studies where cottonseed hulls were the major fiber source, the use of sodium bicarbonate did not improve FCM yield.
Corn silage is an acidic feedstuff, and undoubtedly contributes significantly to the total acid load which must be neutralized by the cow to maintain desirable ruminal pH. In three studies where silage pH was adjusted using sodium bicarbonate from between pH 3.7 and 4.4 to approximately pH 5.0 to 5.8, organic matter intake was increased by 1 to 2 kg/d (18, 19), and average daily gain increased .14 to .17 kg/d over controls (14). Buffering corn silage prior to feeding improved digestibility of DM and NDF over control silage (14). These results suggest that it is the acidity associated with corn silage that elicits the positive response to added sodium bicarbonate. Erdman (5) stated that the consumption of corn silage by a cow with average rumen pH of 6.0 would require the equivalent of 33 grams of sodium bicarbonate/kg of silage DM intake to maintain the rumen pH at 6.0, while fresh forages or hays with pH near 6.0 would require no added sodium bicarbonate to maintain pH.
In a grazing study where cows were offered 1kg concentrate per 3 kg FCM in twice daily portions, control or concentrate containing 1.9% sodium bicarbonate had no effect on intake of concentrate, milk yield, fat or protein percentages (15). Rumen pH was consistently high, as was acetate production. However, a moderate milk fat depression was not improved with the buffer. Given the large quantity of forages consumed in a grazing situation, the benefits from buffers may be small.
Sodium bicarbonate has been used to buffer diets that contain a large proportion of by-products and which are often low in fiber. The rationale behind feeding such diets is often to use fibrous by-products in place of forages. While high in fiber, most by-products have small particle sizes which result in minimal rumination. Thus one might expect cows offered these diets to benefit from additional buffering. A study was conducted where diets were fed containing high forage (49% of DM), or low forage (30 to 35% of DM), with or without corn gluten feed added at 20% of the concentrate, with or without 1% sodium bicarbonate (10). Cows gave more milk for low versus high forage diets (33.7 vs. 30.6 kg/d), and diets containing 20% corn gluten feed improved FCM if they were buffered (33.0 vs. 30.9 kg/d). In addition, digestibility of DM and fiber were improved sharply when buffer was added to the diet. This suggests that fibrous by-products can substitute for a substantial quantity of forage if buffer is added to offset the reduced chewing that results from fine particle size.
Other work with wheat middlings (24) and soyhulls (9) added to low forage diets showed little response to added buffer. Diets containing wheat mids had a high production of VFAs and ruminal pH for all diets was below 5.8 for an extended period
(24). Acid production probably exceeded the capacity for the buffer to be effective. For soyhull containing diets, the authors (9) speculated that the soyhulls had a very fast rate of passage which affected intake and digestion far more than ruminal pH. Thus the buffer had little effect on digestion of the soyhull diets. The fact that there is a variable response to dietary buffers when by-products are fed is not surprising when one considers the considerable variation in feeding characteristics of the many available by-products. Briceno (2) reported large variation in the milk yield which resulted from feeding various by-products. Dry matter intake and milk yield were greater for cottonseed hulls than for other by-products such as sugarcane bagasse and corrugated boxes. Physical form of the feedstuff and digestion characteristics play a large role in how the feedstuff performs, and probably its ruminal effects will determine how effective buffers are in the feeding program. As an example, Staples and Lough (21) reported that an analysis of studies in which cottonseed hulls were fed showed little response to buffering in those diets. Attention to the feedstuff, its fermentability and fiber characteristics will aid in decisions regarding buffer use.A New Concept in Buffering
Much has been said recently about dietary cation-anion difference (DCAD) for dry dairy cows. This concept involves the use of compounds which create a mild metabolic acidosis in the cow at calving which minimizes the incidence of milk fever, and which may enhance milk yield and minimize other disorders. A similar concept has been used in lactating dairy cows, which involves the use of compounds to create an alkaline condition in the cow during lactation, which is the opposite of what occurs with the dry cow. Using the DCAD concept for lactating cows involves using an equation such as the following: DCAD = milliequivalents (meq) of ((Na+ + K+) - Cl-) per 100 gr feed. While the DCAD equation for dry cows incorporates the use of Na, K, S, Cl, and possibly P and strives for a negative value, the equation for lactating cows is simpler, usually using only Na, K, and Cl, and with a highly postive value being desirable. Early poultry and swine work showed that the greatest amount of variation in response was explained by these three elements and most of the lactating cow work has concentrated on these three elements.
The objective of DCAD for lactating cows is to improve the acid-base status of the cow. To alter the DCAD, buffers such as sodium bicarbonate or sodium carbonate, or potassium bicarbonate or potassium carbonate are used, since salts such as NaCl or KCl contain both positive and negative ions and are neutral in the equation. Increasing the value of DCAD in dairy cows increases the volume of blood buffer, which theoretically should increase the resistance to change in pH associated with the production of acids during digestion. Research shows that increasing DCAD improved intake for lactating cows, and that the use of Na+ or K+ to change the equation was effective in improving performance (22, 26). This means that both sodium or potassium buffers were effective in improving DCAD, and can be considered for use on a cost basis.
A review of a large number of mineral studies showed that DMI and milk yield peaked when the DCAD was in the range of 30 to 45 meq ((Na+ + K+) -Cl-) [17]. This is consistent with other studies, and may be another reason that dietary buffers are beneficial. The NRC (13) guidelines for minimum requirements of Na (.18%), K (.9%) and Cl (.25%) yields a DCAD of 23.8 meq per 100 gr feed. The addition of 1% sodium bicarbonate would add approximately .12% Na to the diet, increasing total Na to .3%, and bringing DCAD to 29 meq per 100 gr feed. With buffers and high K forages, it will be relatively easy to bring DCAD into the desirable range.
The DCAD concept needs more research to determine the most desirable range for the lactating cow. However, the results seen are consistent with other research data which indicate that the cow benefits from buffering at the ruminal level as well as the physiologic level. More work is needed in this area.
Summary
Buffers, like many other feed additives, are only beneficial when there is a need. Positive results can occur when the farm situation favors buffers. Buffers are favored when the following situations exist:
References